

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186318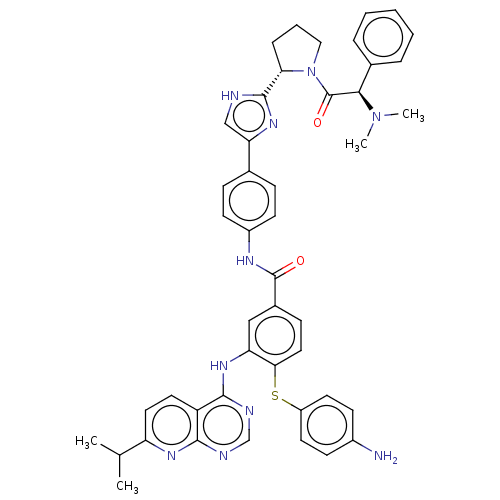 (US9163017, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186343 (US9163017, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186342 (US9163017, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186339 (US9163017, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186338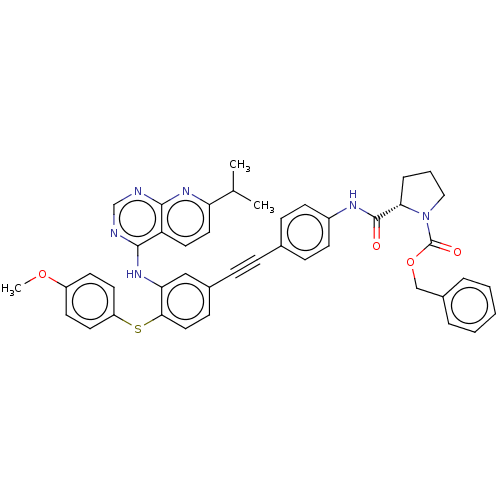 (US9163017, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186337 (US9163017, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186336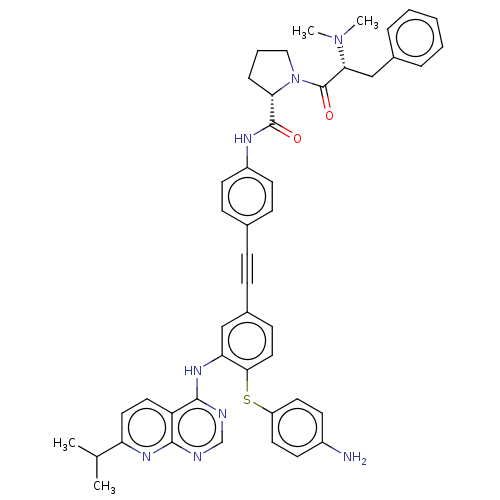 (US9163017, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186335 (US9163017, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186334 (US9163017, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186333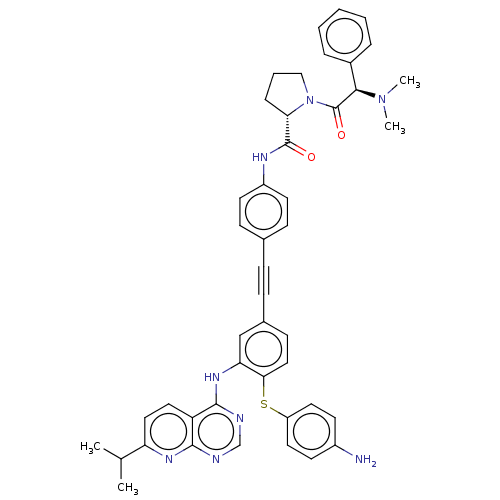 (US9163017, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186332 (US9163017, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186331 (US9163017, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186324 (US9163017, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506590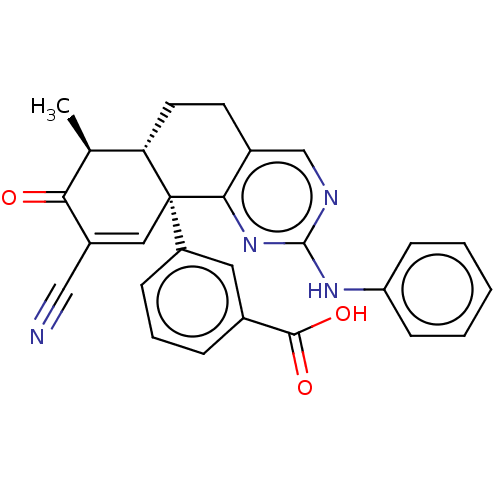 (CHEMBL4593992) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186340 (US9163017, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186341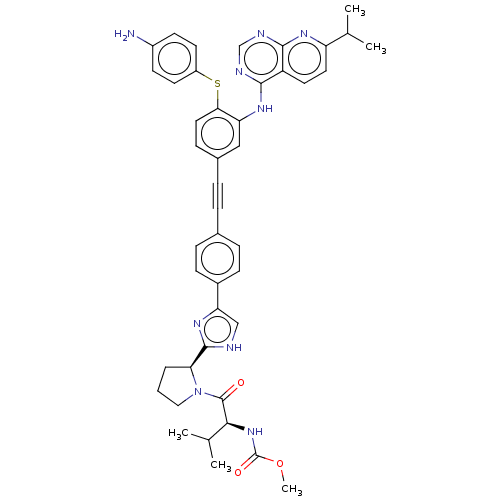 (US9163017, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186305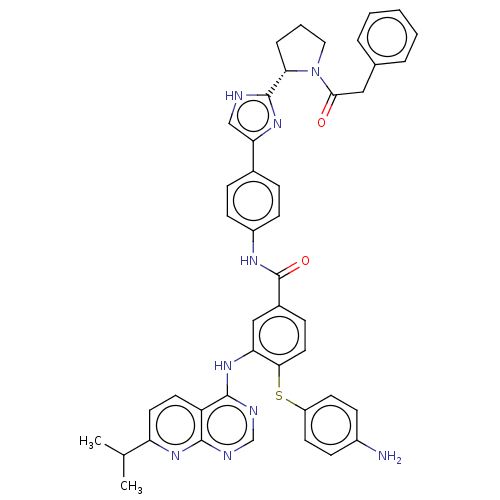 (US9163017, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM186329 (US9163017, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ABBVIE INC. US Patent | Assay Description The inhibitory effects of the compounds of the invention on HCV replication can be determined by measuring activity of the luciferase reporter gene. ... | US Patent US9163017 (2015) BindingDB Entry DOI: 10.7270/Q2F47MX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506594 (CHEMBL4556998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506592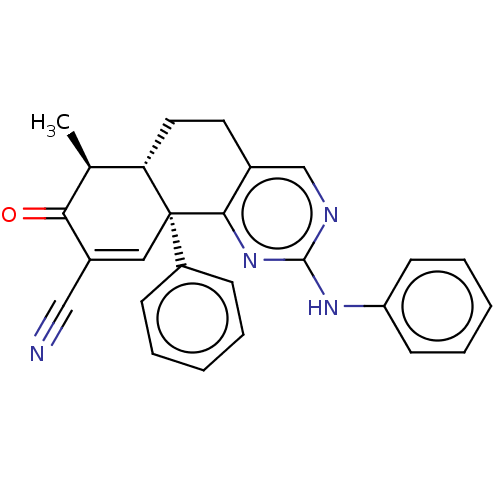 (CHEMBL4535154) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506585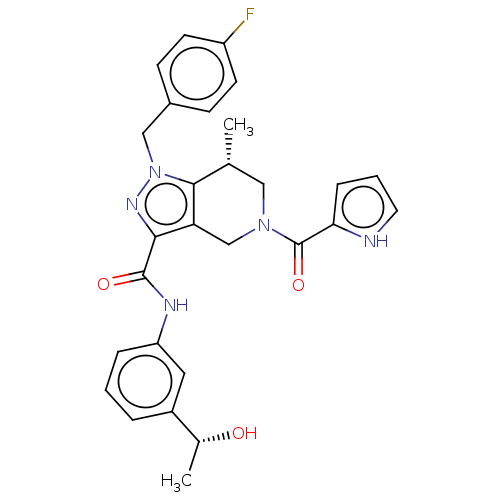 (CHEMBL4515358) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506593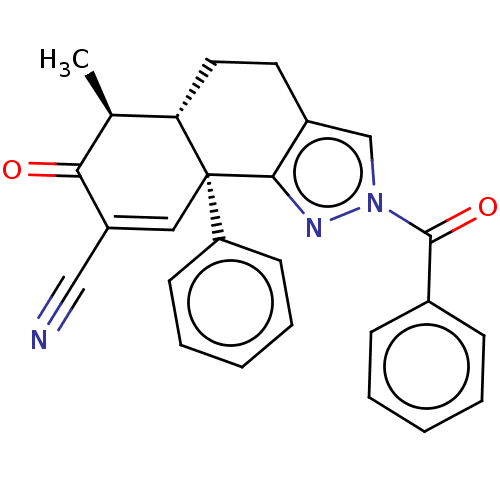 (CHEMBL4540440) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506584 (CHEMBL221360) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506591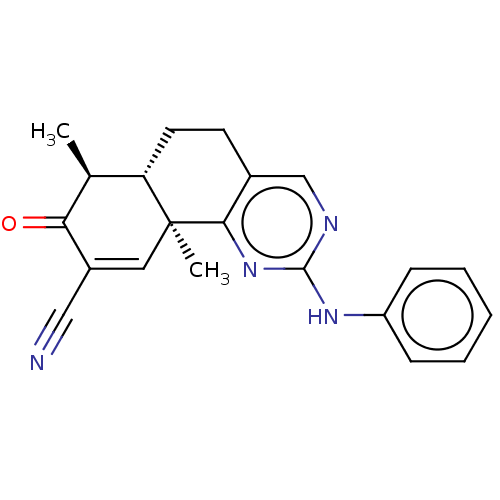 (CHEMBL4548727) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506589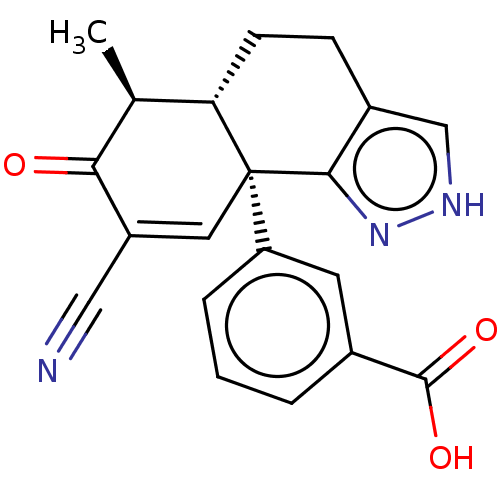 (CHEMBL4549554) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506587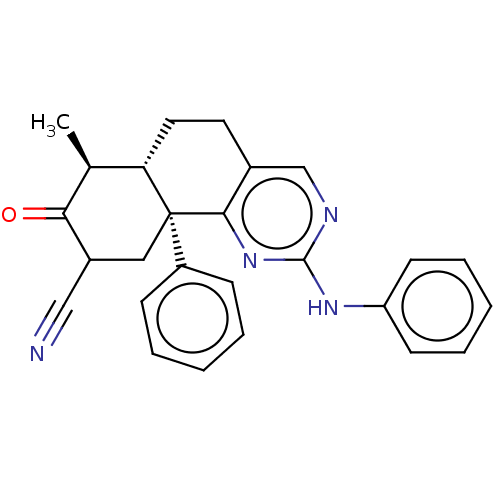 (CHEMBL4551778) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506586 (CHEMBL4459125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM50065970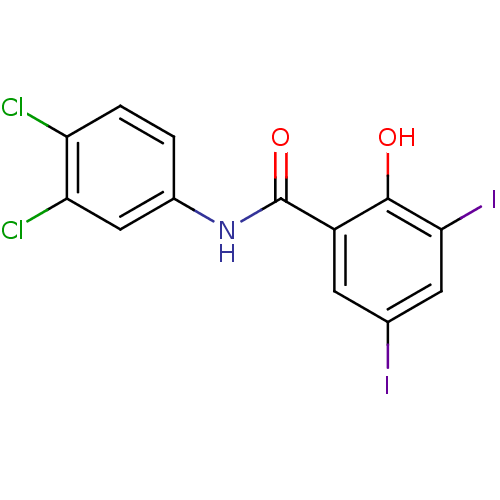 (CHEMBL57656 | N-(3,4-Dichloro-phenyl)-2-hydroxy-3,...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV BK NS5B polymerase | Bioorg Med Chem Lett 18: 3173-7 (2008) Article DOI: 10.1016/j.bmcl.2008.04.068 BindingDB Entry DOI: 10.7270/Q2SQ905N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506583 (CHEMBL4562890) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50506588 (CHEMBL4467995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal 8-His tagged wild type human IDH1 (1 to 414 residues) expressed in Escherichia coli BL21(DE3)-T1R preincubated for 15 mins u... | J Med Chem 61: 6647-6657 (2018) Article DOI: 10.1021/acs.jmedchem.8b00305 BindingDB Entry DOI: 10.7270/Q2RJ4NR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453078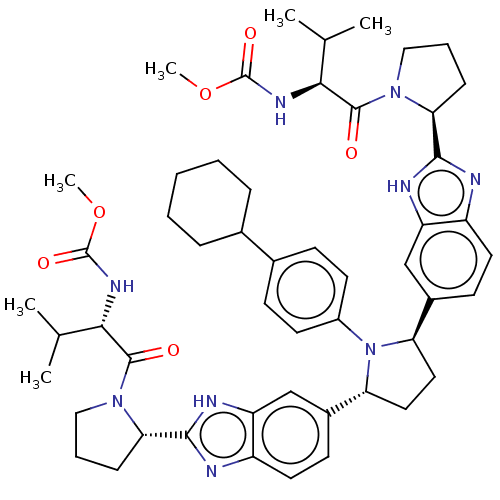 (CHEMBL4215254) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2b assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453100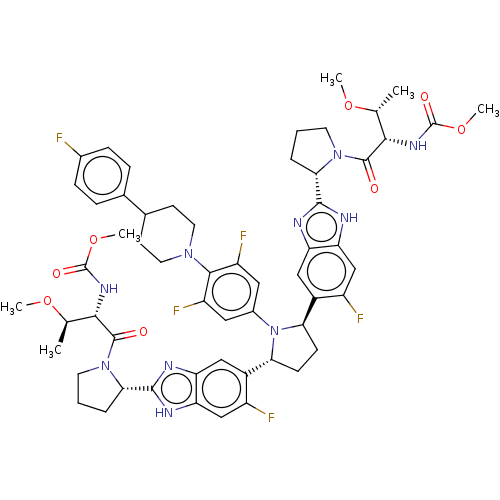 (A-1325912.0 | ABT-530 | Pibrentasvir) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00190 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2b assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453101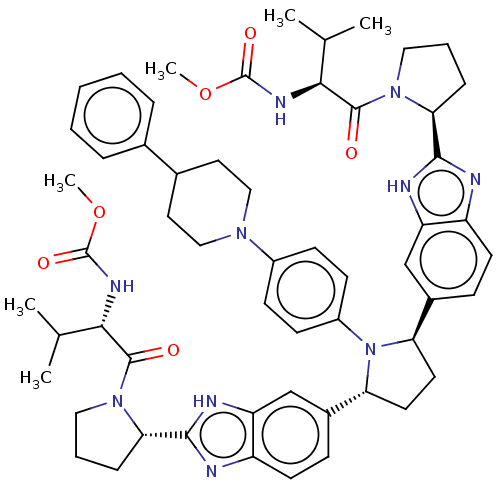 (CHEMBL4208591) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.481 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 3a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453102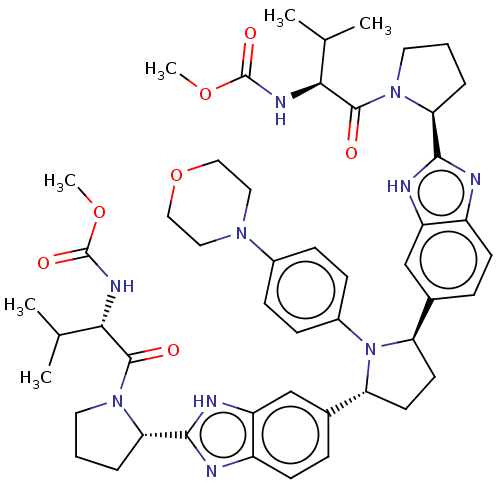 (CHEMBL4206465) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 4a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453103 (CHEMBL4218872) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 5a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50453104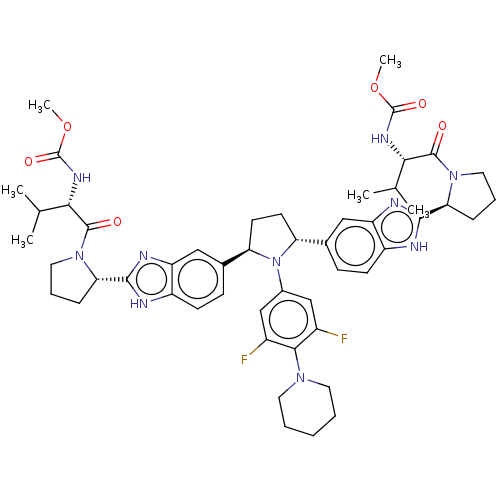 (CHEMBL3915742) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1a H77 assessed as decrease in viral replication after 3 days in presence of 40% human plasma b... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453105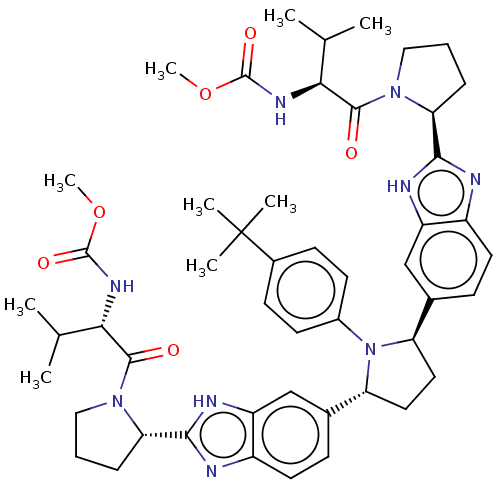 (CHEMBL3960757) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 4a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453105 (CHEMBL3960757) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.152 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453106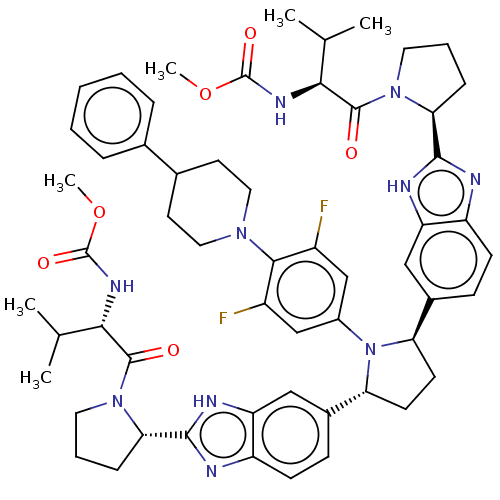 (CHEMBL4215923) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days in presence of 40% human plasma ... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453107 (CHEMBL4215241) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days in presence of 40% human plasma ... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50453108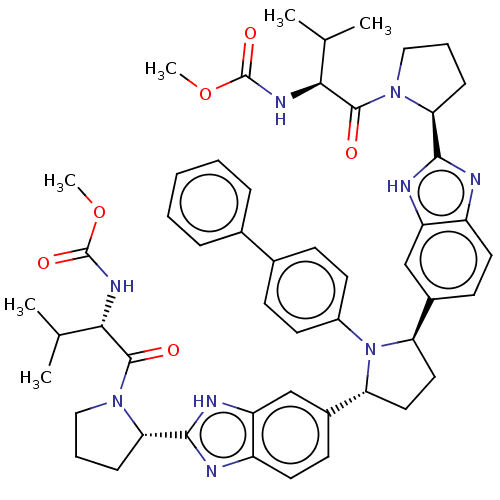 (CHEMBL4202479) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1a H77 assessed as decrease in viral replication after 3 days in presence of 40% human plasma b... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453109 (CHEMBL4205352) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days by luciferase reporter gene assa... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453107 (CHEMBL4215241) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days by luciferase reporter gene assa... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50453110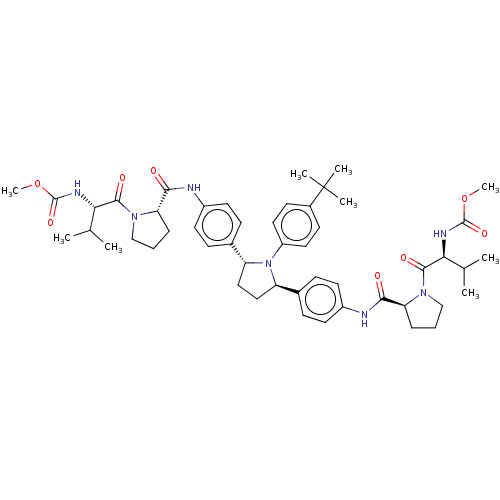 (CHEMBL3127327) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.136 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1a H77 assessed as decrease in viral replication after 3 days by luciferase reporter gene assay | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453111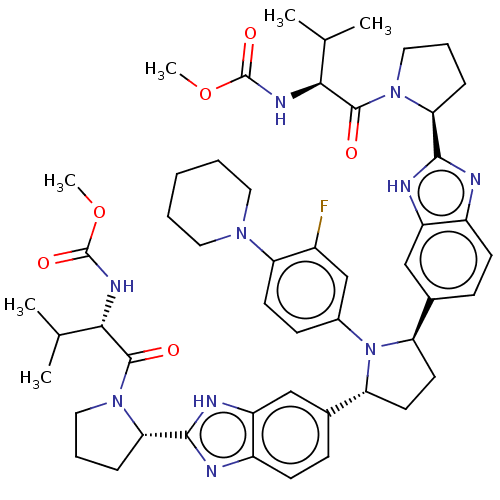 (CHEMBL4208073) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453112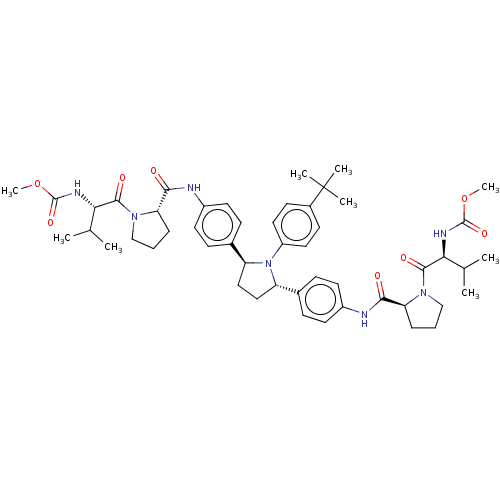 (ABT-267 | CHEBI:85183 | Ombitasvir) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00430 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2b assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453113 (CHEMBL4210254) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 3a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453114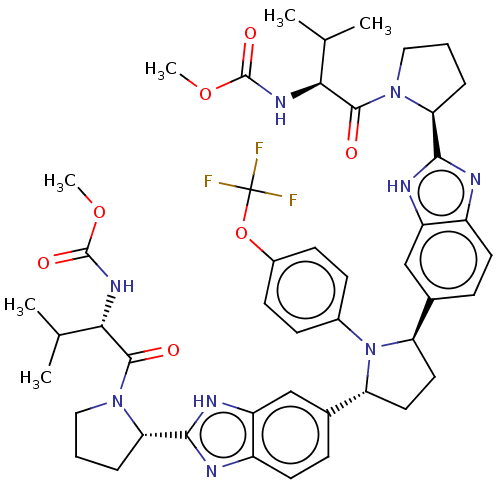 (CHEMBL4211348) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 4a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453113 (CHEMBL4210254) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 5a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 246 total ) | Next | Last >> |