 Found 137 hits with Last Name = 'premnath' and Initial = 'pn'
Found 137 hits with Last Name = 'premnath' and Initial = 'pn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182728
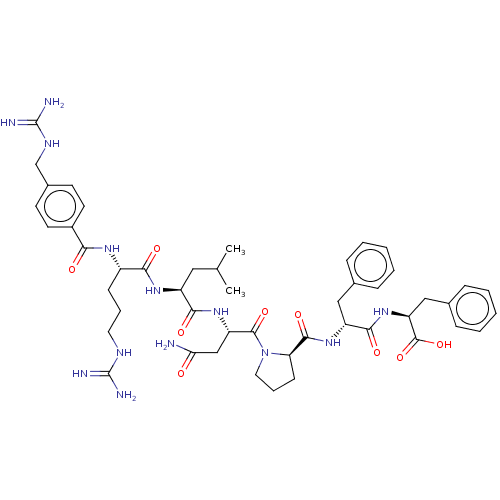
(CHEMBL3818393)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C48H65N13O9/c1-28(2)23-34(57-41(64)33(15-9-21-54-47(50)51)56-40(63)32-19-17-31(18-20-32)27-55-48(52)53)42(65)59-36(26-39(49)62)45(68)61-22-10-16-38(61)44(67)58-35(24-29-11-5-3-6-12-29)43(66)60-37(46(69)70)25-30-13-7-4-8-14-30/h3-8,11-14,17-20,28,33-38H,9-10,15-16,21-27H2,1-2H3,(H2,49,62)(H,56,63)(H,57,64)(H,58,67)(H,59,65)(H,60,66)(H,69,70)(H4,50,51,54)(H4,52,53,55)/t33-,34-,35+,36-,37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50444660

(CHEMBL3098662)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(F)cc2)-[#6](-[#8])=O)cc1-[#8]-[#6]-[#6] |r| Show InChI InChI=1S/C37H53FN8O9/c1-5-54-29-14-11-23(18-30(29)55-6-2)19-32(48)43-25(8-7-15-42-37(40)41)33(49)44-26(16-21(3)4)34(50)45-27(20-31(39)47)35(51)46-28(36(52)53)17-22-9-12-24(38)13-10-22/h9-14,18,21,25-28H,5-8,15-17,19-20H2,1-4H3,(H2,39,47)(H,43,48)(H,44,49)(H,45,50)(H,46,51)(H,52,53)(H4,40,41,42)/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK4/cyclin D1 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate after 45 mins by fluorescenc... |
Bioorg Med Chem 22: 616-22 (2013)
Article DOI: 10.1016/j.bmc.2013.10.039
BindingDB Entry DOI: 10.7270/Q2416ZHS |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490475
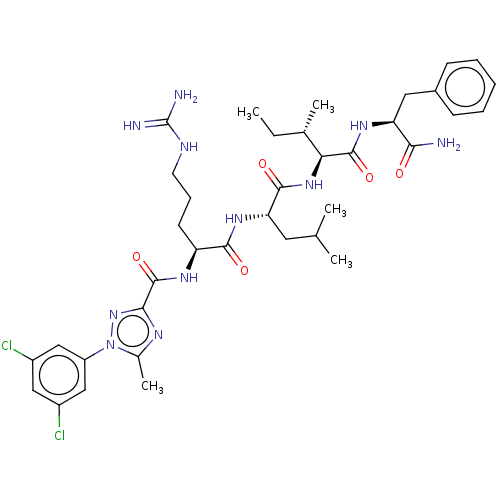
(CHEMBL2326552)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1cc(Cl)cc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H51Cl2N11O5/c1-6-21(4)30(35(54)46-28(31(40)51)16-23-11-8-7-9-12-23)48-34(53)29(15-20(2)3)47-33(52)27(13-10-14-43-37(41)42)45-36(55)32-44-22(5)50(49-32)26-18-24(38)17-25(39)19-26/h7-9,11-12,17-21,27-30H,6,10,13-16H2,1-5H3,(H2,40,51)(H,45,55)(H,46,54)(H,47,52)(H,48,53)(H4,41,42,43)/t21-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182655

(CHEMBL3818194)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)c(O)c1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H59N9O7/c1-5-25(4)33(37(53)46-31(38(54)55)21-26-10-7-6-8-11-26)47-36(52)30(20-24(2)3)45-35(51)29(12-9-15-43-39(40)41)44-34(50)27-13-14-28(32(49)22-27)23-48-18-16-42-17-19-48/h6-8,10-11,13-14,22,24-25,29-31,33,42,49H,5,9,12,15-21,23H2,1-4H3,(H,44,50)(H,45,51)(H,46,53)(H,47,52)(H,54,55)(H4,40,41,43)/t25-,29-,30-,31-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182654
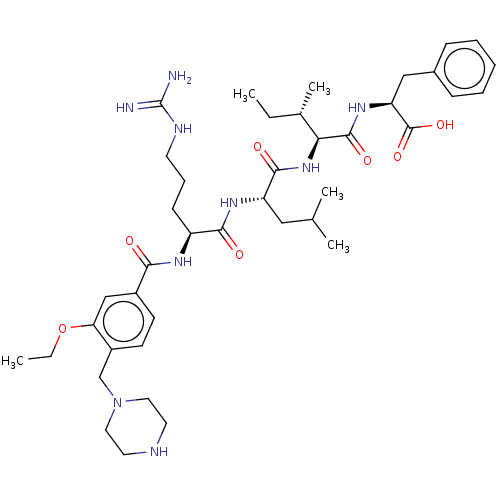
(CHEMBL3819050)Show SMILES CCOc1cc(ccc1CN1CCNCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H63N9O7/c1-6-27(5)35(39(54)48-33(40(55)56)23-28-12-9-8-10-13-28)49-38(53)32(22-26(3)4)47-37(52)31(14-11-17-45-41(42)43)46-36(51)29-15-16-30(34(24-29)57-7-2)25-50-20-18-44-19-21-50/h8-10,12-13,15-16,24,26-27,31-33,35,44H,6-7,11,14,17-23,25H2,1-5H3,(H,46,51)(H,47,52)(H,48,54)(H,49,53)(H,55,56)(H4,42,43,45)/t27-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444660

(CHEMBL3098662)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(F)cc2)-[#6](-[#8])=O)cc1-[#8]-[#6]-[#6] |r| Show InChI InChI=1S/C37H53FN8O9/c1-5-54-29-14-11-23(18-30(29)55-6-2)19-32(48)43-25(8-7-15-42-37(40)41)33(49)44-26(16-21(3)4)34(50)45-27(20-31(39)47)35(51)46-28(36(52)53)17-22-9-12-24(38)13-10-22/h9-14,18,21,25-28H,5-8,15-17,19-20H2,1-4H3,(H2,39,47)(H,43,48)(H,44,49)(H,45,50)(H,46,51)(H,52,53)(H4,40,41,42)/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/cyclin A using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate after 45 mins by fluorescence pola... |
Bioorg Med Chem 22: 616-22 (2013)
Article DOI: 10.1016/j.bmc.2013.10.039
BindingDB Entry DOI: 10.7270/Q2416ZHS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182730
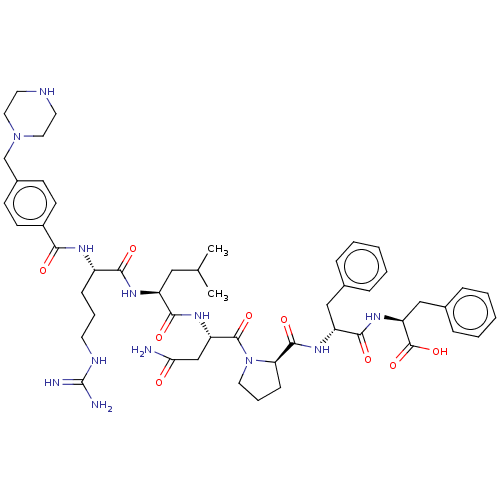
(CHEMBL3818746)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C51H70N12O9/c1-32(2)27-38(58-45(66)37(15-9-21-56-51(53)54)57-44(65)36-19-17-35(18-20-36)31-62-25-22-55-23-26-62)46(67)60-40(30-43(52)64)49(70)63-24-10-16-42(63)48(69)59-39(28-33-11-5-3-6-12-33)47(68)61-41(50(71)72)29-34-13-7-4-8-14-34/h3-8,11-14,17-20,32,37-42,55H,9-10,15-16,21-31H2,1-2H3,(H2,52,64)(H,57,65)(H,58,66)(H,59,69)(H,60,67)(H,61,68)(H,71,72)(H4,53,54,56)/t37-,38-,39+,40-,41-,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490482
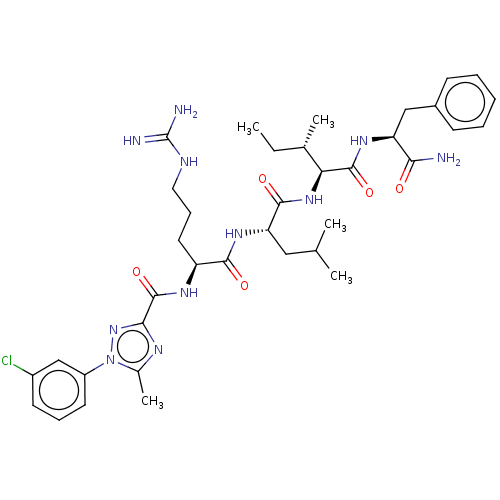
(CHEMBL2326554)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H52ClN11O5/c1-6-22(4)30(35(53)45-28(31(39)50)19-24-12-8-7-9-13-24)47-34(52)29(18-21(2)3)46-33(51)27(16-11-17-42-37(40)41)44-36(54)32-43-23(5)49(48-32)26-15-10-14-25(38)20-26/h7-10,12-15,20-22,27-30H,6,11,16-19H2,1-5H3,(H2,39,50)(H,44,54)(H,45,53)(H,46,51)(H,47,52)(H4,40,41,42)/t22-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182654

(CHEMBL3819050)Show SMILES CCOc1cc(ccc1CN1CCNCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H63N9O7/c1-6-27(5)35(39(54)48-33(40(55)56)23-28-12-9-8-10-13-28)49-38(53)32(22-26(3)4)47-37(52)31(14-11-17-45-41(42)43)46-36(51)29-15-16-30(34(24-29)57-7-2)25-50-20-18-44-19-21-50/h8-10,12-13,15-16,24,26-27,31-33,35,44H,6-7,11,14,17-23,25H2,1-5H3,(H,46,51)(H,47,52)(H,48,54)(H,49,53)(H,55,56)(H4,42,43,45)/t27-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182722
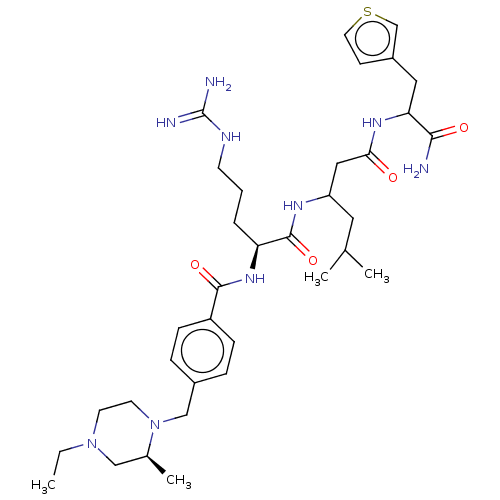
(CHEMBL3818751)Show SMILES CCN1CCN(Cc2ccc(cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)NC(Cc2ccsc2)C(N)=O)[C@@H](C)C1 |r| Show InChI InChI=1S/C35H55N9O4S/c1-5-43-14-15-44(24(4)20-43)21-25-8-10-27(11-9-25)33(47)42-29(7-6-13-39-35(37)38)34(48)40-28(17-23(2)3)19-31(45)41-30(32(36)46)18-26-12-16-49-22-26/h8-12,16,22-24,28-30H,5-7,13-15,17-21H2,1-4H3,(H2,36,46)(H,40,48)(H,41,45)(H,42,47)(H4,37,38,39)/t24-,28?,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182653
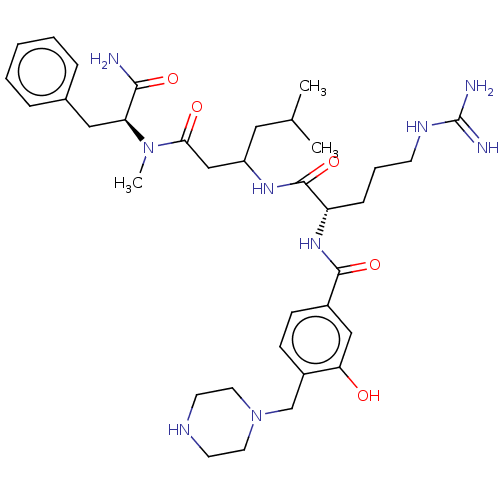
(CHEMBL3818708)Show SMILES CC(C)CC(CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)c(O)c1 |r| Show InChI InChI=1S/C35H53N9O5/c1-23(2)18-27(21-31(46)43(3)29(32(36)47)19-24-8-5-4-6-9-24)41-34(49)28(10-7-13-40-35(37)38)42-33(48)25-11-12-26(30(45)20-25)22-44-16-14-39-15-17-44/h4-6,8-9,11-12,20,23,27-29,39,45H,7,10,13-19,21-22H2,1-3H3,(H2,36,47)(H,41,49)(H,42,48)(H4,37,38,40)/t27?,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182727
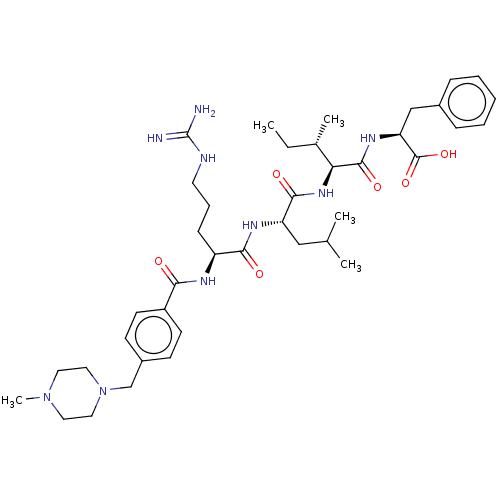
(CHEMBL3819143)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H61N9O6/c1-6-27(4)34(38(53)46-33(39(54)55)24-28-11-8-7-9-12-28)47-37(52)32(23-26(2)3)45-36(51)31(13-10-18-43-40(41)42)44-35(50)30-16-14-29(15-17-30)25-49-21-19-48(5)20-22-49/h7-9,11-12,14-17,26-27,31-34H,6,10,13,18-25H2,1-5H3,(H,44,50)(H,45,51)(H,46,53)(H,47,52)(H,54,55)(H4,41,42,43)/t27-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182724
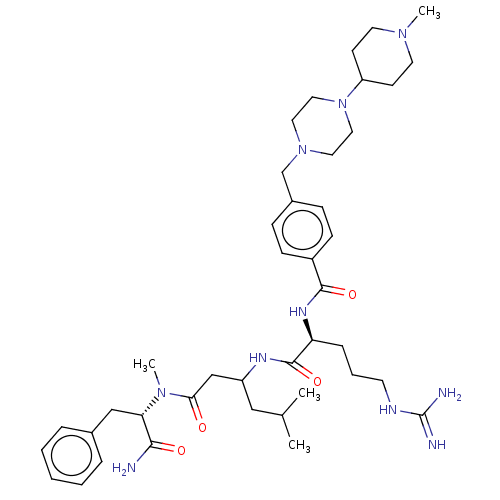
(CHEMBL3818170)Show SMILES CC(C)CC(CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCN(CC2)C2CCN(C)CC2)cc1 |r| Show InChI InChI=1S/C41H64N10O4/c1-29(2)25-33(27-37(52)49(4)36(38(42)53)26-30-9-6-5-7-10-30)46-40(55)35(11-8-18-45-41(43)44)47-39(54)32-14-12-31(13-15-32)28-50-21-23-51(24-22-50)34-16-19-48(3)20-17-34/h5-7,9-10,12-15,29,33-36H,8,11,16-28H2,1-4H3,(H2,42,53)(H,46,55)(H,47,54)(H4,43,44,45)/t33?,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182721
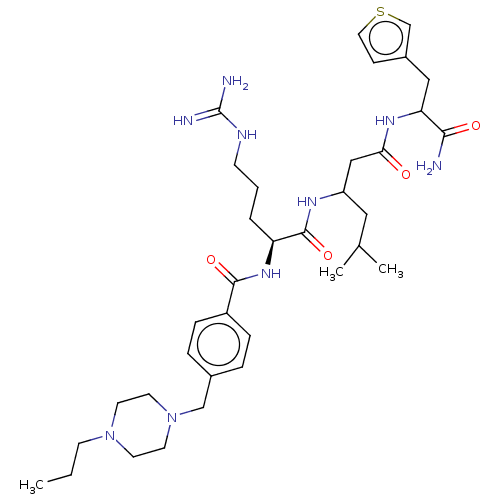
(CHEMBL3818288)Show SMILES CCCN1CCN(Cc2ccc(cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)NC(Cc2ccsc2)C(N)=O)CC1 |r| Show InChI InChI=1S/C35H55N9O4S/c1-4-13-43-14-16-44(17-15-43)22-25-7-9-27(10-8-25)33(47)42-29(6-5-12-39-35(37)38)34(48)40-28(19-24(2)3)21-31(45)41-30(32(36)46)20-26-11-18-49-23-26/h7-11,18,23-24,28-30H,4-6,12-17,19-22H2,1-3H3,(H2,36,46)(H,40,48)(H,41,45)(H,42,47)(H4,37,38,39)/t28?,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182651
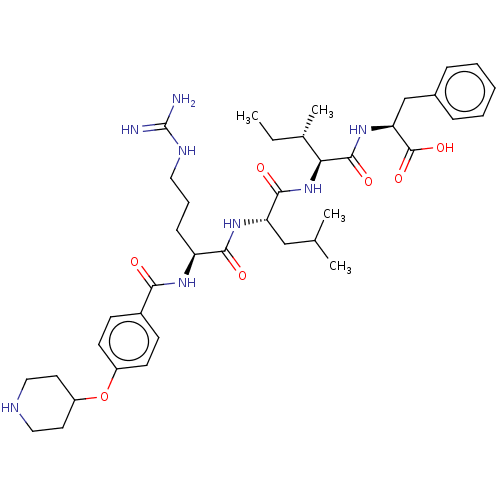
(CHEMBL3819101)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(OC2CCNCC2)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H58N8O7/c1-5-25(4)33(37(51)46-32(38(52)53)23-26-10-7-6-8-11-26)47-36(50)31(22-24(2)3)45-35(49)30(12-9-19-43-39(40)41)44-34(48)27-13-15-28(16-14-27)54-29-17-20-42-21-18-29/h6-8,10-11,13-16,24-25,29-33,42H,5,9,12,17-23H2,1-4H3,(H,44,48)(H,45,49)(H,46,51)(H,47,50)(H,52,53)(H4,40,41,43)/t25-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182708
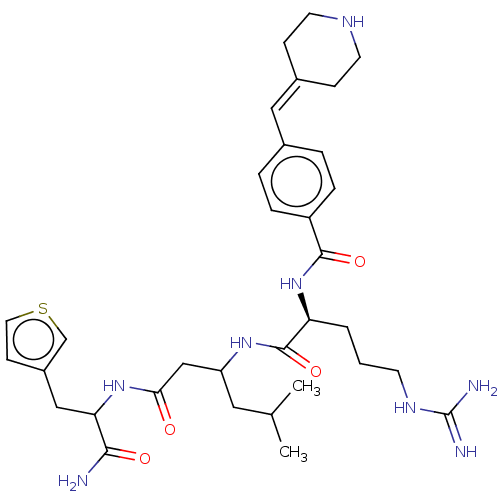
(CHEMBL3819005)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6](-[#6]-[#6](=O)-[#7]-[#6](-[#6]-c1ccsc1)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-c1ccc(\[#6]=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C33H48N8O4S/c1-21(2)16-26(19-29(42)40-28(30(34)43)18-24-11-15-46-20-24)39-32(45)27(4-3-12-38-33(35)36)41-31(44)25-7-5-22(6-8-25)17-23-9-13-37-14-10-23/h5-8,11,15,17,20-21,26-28,37H,3-4,9-10,12-14,16,18-19H2,1-2H3,(H2,34,43)(H,39,45)(H,40,42)(H,41,44)(H4,35,36,38)/t26?,27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182720
(CHEMBL3819344)Show SMILES CCCN1CCN(Cc2ccc(cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)NC(Cc2ccsc2)C(N)=O)[C@@H](C)C1 |r| Show InChI InChI=1S/C36H57N9O4S/c1-5-14-44-15-16-45(25(4)21-44)22-26-8-10-28(11-9-26)34(48)43-30(7-6-13-40-36(38)39)35(49)41-29(18-24(2)3)20-32(46)42-31(33(37)47)19-27-12-17-50-23-27/h8-12,17,23-25,29-31H,5-7,13-16,18-22H2,1-4H3,(H2,37,47)(H,41,49)(H,42,46)(H,43,48)(H4,38,39,40)/t25-,29?,30-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182649
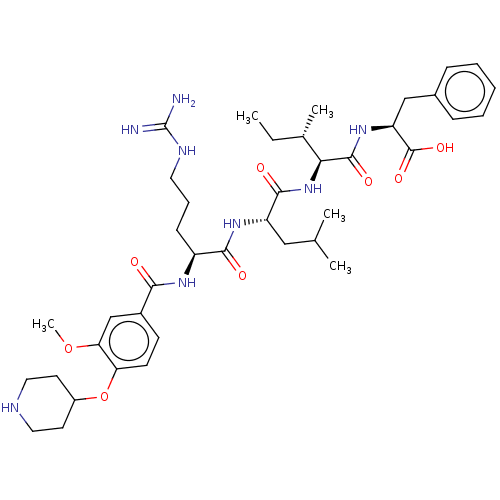
(CHEMBL3817868)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(OC2CCNCC2)c(OC)c1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H60N8O8/c1-6-25(4)34(38(52)47-31(39(53)54)22-26-11-8-7-9-12-26)48-37(51)30(21-24(2)3)46-36(50)29(13-10-18-44-40(41)42)45-35(49)27-14-15-32(33(23-27)55-5)56-28-16-19-43-20-17-28/h7-9,11-12,14-15,23-25,28-31,34,43H,6,10,13,16-22H2,1-5H3,(H,45,49)(H,46,50)(H,47,52)(H,48,51)(H,53,54)(H4,41,42,44)/t25-,29-,30-,31-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182655

(CHEMBL3818194)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)c(O)c1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H59N9O7/c1-5-25(4)33(37(53)46-31(38(54)55)21-26-10-7-6-8-11-26)47-36(52)30(20-24(2)3)45-35(51)29(12-9-15-43-39(40)41)44-34(50)27-13-14-28(32(49)22-27)23-48-18-16-42-17-19-48/h6-8,10-11,13-14,22,24-25,29-31,33,42,49H,5,9,12,15-21,23H2,1-4H3,(H,44,50)(H,45,51)(H,46,53)(H,47,52)(H,54,55)(H4,40,41,43)/t25-,29-,30-,31-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182727

(CHEMBL3819143)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H61N9O6/c1-6-27(4)34(38(53)46-33(39(54)55)24-28-11-8-7-9-12-28)47-37(52)32(23-26(2)3)45-36(51)31(13-10-18-43-40(41)42)44-35(50)30-16-14-29(15-17-30)25-49-21-19-48(5)20-22-49/h7-9,11-12,14-17,26-27,31-34H,6,10,13,18-25H2,1-5H3,(H,44,50)(H,45,51)(H,46,53)(H,47,52)(H,54,55)(H4,41,42,43)/t27-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490474
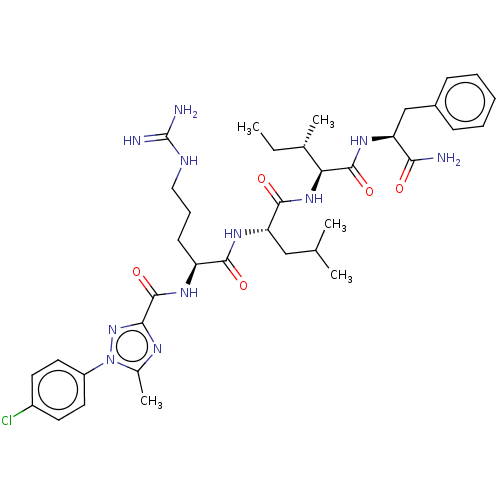
(CHEMBL2326553)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H52ClN11O5/c1-6-22(4)30(35(53)45-28(31(39)50)20-24-11-8-7-9-12-24)47-34(52)29(19-21(2)3)46-33(51)27(13-10-18-42-37(40)41)44-36(54)32-43-23(5)49(48-32)26-16-14-25(38)15-17-26/h7-9,11-12,14-17,21-22,27-30H,6,10,13,18-20H2,1-5H3,(H2,39,50)(H,44,54)(H,45,53)(H,46,51)(H,47,52)(H4,40,41,42)/t22-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182731
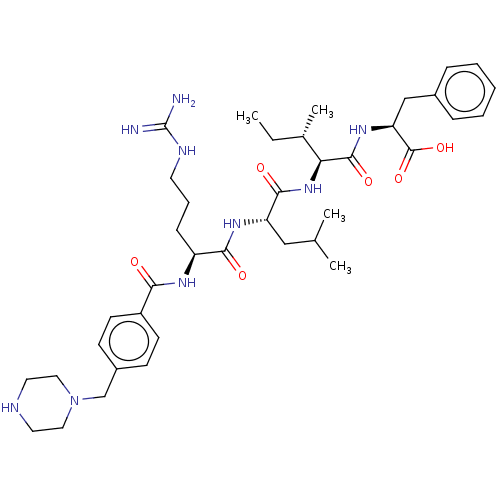
(CHEMBL3817924)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H59N9O6/c1-5-26(4)33(37(52)46-32(38(53)54)23-27-10-7-6-8-11-27)47-36(51)31(22-25(2)3)45-35(50)30(12-9-17-43-39(40)41)44-34(49)29-15-13-28(14-16-29)24-48-20-18-42-19-21-48/h6-8,10-11,13-16,25-26,30-33,42H,5,9,12,17-24H2,1-4H3,(H,44,49)(H,45,50)(H,46,52)(H,47,51)(H,53,54)(H4,40,41,43)/t26-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182653

(CHEMBL3818708)Show SMILES CC(C)CC(CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)c(O)c1 |r| Show InChI InChI=1S/C35H53N9O5/c1-23(2)18-27(21-31(46)43(3)29(32(36)47)19-24-8-5-4-6-9-24)41-34(49)28(10-7-13-40-35(37)38)42-33(48)25-11-12-26(30(45)20-25)22-44-16-14-39-15-17-44/h4-6,8-9,11-12,20,23,27-29,39,45H,7,10,13-19,21-22H2,1-3H3,(H2,36,47)(H,41,49)(H,42,48)(H4,37,38,40)/t27?,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490481
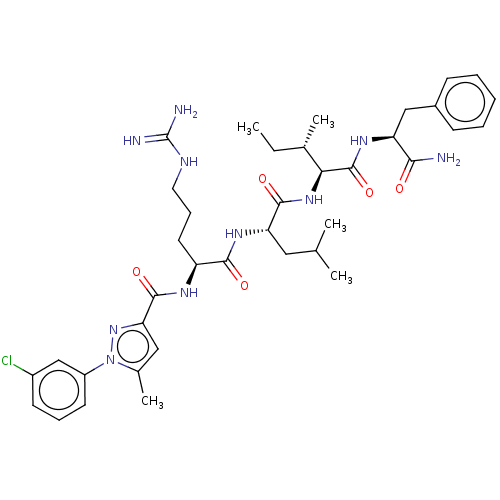
(CHEMBL2326558)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(C)n(n1)-c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C38H53ClN10O5/c1-6-23(4)32(37(54)45-29(33(40)50)20-25-12-8-7-9-13-25)47-35(52)30(18-22(2)3)46-34(51)28(16-11-17-43-38(41)42)44-36(53)31-19-24(5)49(48-31)27-15-10-14-26(39)21-27/h7-10,12-15,19,21-23,28-30,32H,6,11,16-18,20H2,1-5H3,(H2,40,50)(H,44,53)(H,45,54)(H,46,51)(H,47,52)(H4,41,42,43)/t23-,28-,29-,30-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50490474

(CHEMBL2326553)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H52ClN11O5/c1-6-22(4)30(35(53)45-28(31(39)50)20-24-11-8-7-9-12-24)47-34(52)29(19-21(2)3)46-33(51)27(13-10-18-42-37(40)41)44-36(54)32-43-23(5)49(48-32)26-16-14-25(38)15-17-26/h7-9,11-12,14-17,21-22,27-30H,6,10,13,18-20H2,1-5H3,(H2,39,50)(H,44,54)(H,45,53)(H,46,51)(H,47,52)(H4,40,41,42)/t22-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK4/Cyclin D1 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182730

(CHEMBL3818746)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C51H70N12O9/c1-32(2)27-38(58-45(66)37(15-9-21-56-51(53)54)57-44(65)36-19-17-35(18-20-36)31-62-25-22-55-23-26-62)46(67)60-40(30-43(52)64)49(70)63-24-10-16-42(63)48(69)59-39(28-33-11-5-3-6-12-33)47(68)61-41(50(71)72)29-34-13-7-4-8-14-34/h3-8,11-14,17-20,32,37-42,55H,9-10,15-16,21-31H2,1-2H3,(H2,52,64)(H,57,65)(H,58,66)(H,59,69)(H,60,67)(H,61,68)(H,71,72)(H4,53,54,56)/t37-,38-,39+,40-,41-,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182731

(CHEMBL3817924)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNCC2)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H59N9O6/c1-5-26(4)33(37(52)46-32(38(53)54)23-27-10-7-6-8-11-27)47-36(51)31(22-25(2)3)45-35(50)30(12-9-17-43-39(40)41)44-34(49)29-15-13-28(14-16-29)24-48-20-18-42-19-21-48/h6-8,10-11,13-16,25-26,30-33,42H,5,9,12,17-24H2,1-4H3,(H,44,49)(H,45,50)(H,46,52)(H,47,51)(H,53,54)(H4,40,41,43)/t26-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182737

(CHEMBL3819426)Show SMILES CC(C)CC(CC(=O)NC(Cc1ccsc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNC[C@@H]2C)c(O)c1 |r| Show InChI InChI=1S/C33H51N9O5S/c1-20(2)13-25(16-29(44)40-27(30(34)45)14-22-8-12-48-19-22)39-32(47)26(5-4-9-38-33(35)36)41-31(46)23-6-7-24(28(43)15-23)18-42-11-10-37-17-21(42)3/h6-8,12,15,19-21,25-27,37,43H,4-5,9-11,13-14,16-18H2,1-3H3,(H2,34,45)(H,39,47)(H,40,44)(H,41,46)(H4,35,36,38)/t21-,25?,26-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182726
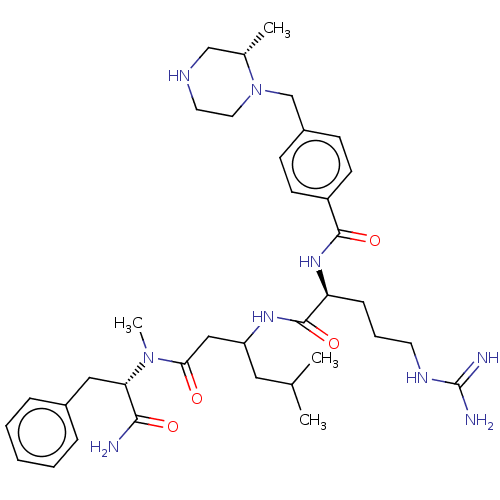
(CHEMBL3818647)Show SMILES CC(C)CC(CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNC[C@@H]2C)cc1 |r| Show InChI InChI=1S/C36H55N9O4/c1-24(2)19-29(21-32(46)44(4)31(33(37)47)20-26-9-6-5-7-10-26)42-35(49)30(11-8-16-41-36(38)39)43-34(48)28-14-12-27(13-15-28)23-45-18-17-40-22-25(45)3/h5-7,9-10,12-15,24-25,29-31,40H,8,11,16-23H2,1-4H3,(H2,37,47)(H,42,49)(H,43,48)(H4,38,39,41)/t25-,29?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490484
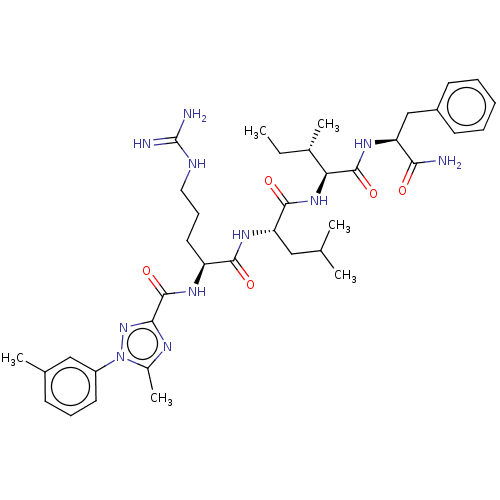
(CHEMBL2326555)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1cccc(C)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C38H55N11O5/c1-7-24(5)31(36(53)45-29(32(39)50)21-26-14-9-8-10-15-26)47-35(52)30(19-22(2)3)46-34(51)28(17-12-18-42-38(40)41)44-37(54)33-43-25(6)49(48-33)27-16-11-13-23(4)20-27/h8-11,13-16,20,22,24,28-31H,7,12,17-19,21H2,1-6H3,(H2,39,50)(H,44,54)(H,45,53)(H,46,51)(H,47,52)(H4,40,41,42)/t24-,28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
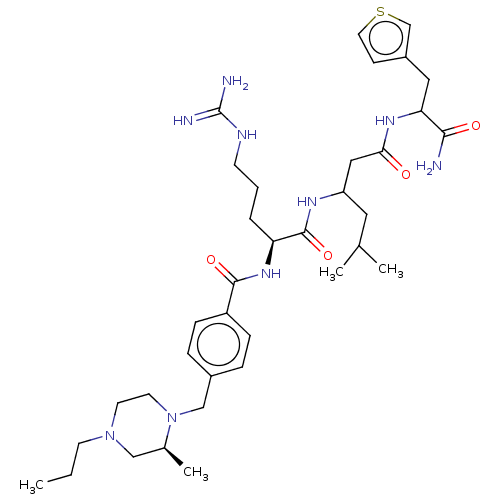
(Homo sapiens (Human)) | BDBM50182728

(CHEMBL3818393)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C48H65N13O9/c1-28(2)23-34(57-41(64)33(15-9-21-54-47(50)51)56-40(63)32-19-17-31(18-20-32)27-55-48(52)53)42(65)59-36(26-39(49)62)45(68)61-22-10-16-38(61)44(67)58-35(24-29-11-5-3-6-12-29)43(66)60-37(46(69)70)25-30-13-7-4-8-14-30/h3-8,11-14,17-20,28,33-38H,9-10,15-16,21-27H2,1-2H3,(H2,49,62)(H,56,63)(H,57,64)(H,58,67)(H,59,65)(H,60,66)(H,69,70)(H4,50,51,54)(H4,52,53,55)/t33-,34-,35+,36-,37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444659

(CHEMBL3098653)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C33H57N11O6/c1-5-20(4)26(30(48)43-25(31(49)50)18-21-11-7-6-8-12-21)44-29(47)24(17-19(2)3)42-28(46)23(14-10-16-40-33(37)38)41-27(45)22(34)13-9-15-39-32(35)36/h6-8,11-12,19-20,22-26H,5,9-10,13-18,34H2,1-4H3,(H,41,45)(H,42,46)(H,43,48)(H,44,47)(H,49,50)(H4,35,36,39)(H4,37,38,40)/t20-,22-,23-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/cyclin A using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate after 45 mins by fluorescence pola... |
Bioorg Med Chem 22: 616-22 (2013)
Article DOI: 10.1016/j.bmc.2013.10.039
BindingDB Entry DOI: 10.7270/Q2416ZHS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50444659

(CHEMBL3098653)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C33H57N11O6/c1-5-20(4)26(30(48)43-25(31(49)50)18-21-11-7-6-8-12-21)44-29(47)24(17-19(2)3)42-28(46)23(14-10-16-40-33(37)38)41-27(45)22(34)13-9-15-39-32(35)36/h6-8,11-12,19-20,22-26H,5,9-10,13-18,34H2,1-4H3,(H,41,45)(H,42,46)(H,43,48)(H,44,47)(H,49,50)(H4,35,36,39)(H4,37,38,40)/t20-,22-,23-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK4/cyclin D1 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate after 45 mins by fluorescenc... |
Bioorg Med Chem 22: 616-22 (2013)
Article DOI: 10.1016/j.bmc.2013.10.039
BindingDB Entry DOI: 10.7270/Q2416ZHS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182723
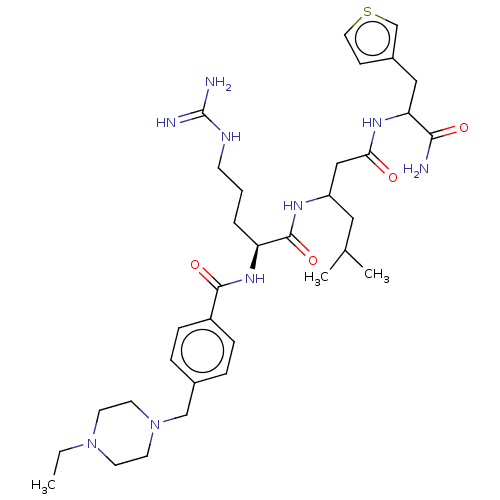
(CHEMBL3818681)Show SMILES CCN1CCN(Cc2ccc(cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)NC(Cc2ccsc2)C(N)=O)CC1 |r| Show InChI InChI=1S/C34H53N9O4S/c1-4-42-13-15-43(16-14-42)21-24-7-9-26(10-8-24)32(46)41-28(6-5-12-38-34(36)37)33(47)39-27(18-23(2)3)20-30(44)40-29(31(35)45)19-25-11-17-48-22-25/h7-11,17,22-23,27-29H,4-6,12-16,18-21H2,1-3H3,(H2,35,45)(H,39,47)(H,40,44)(H,41,46)(H4,36,37,38)/t27?,28-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490466
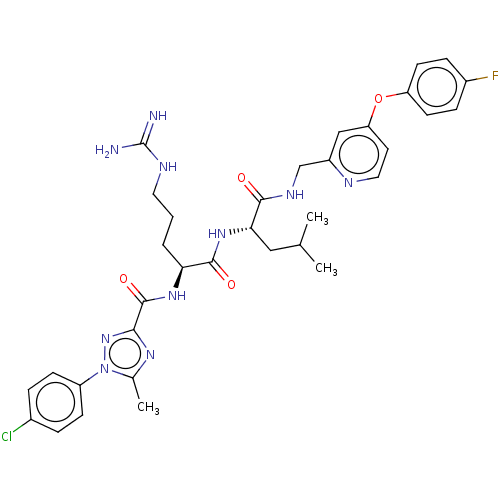
(CHEMBL2326572)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1ccc(Cl)cc1)C(=O)NCc1cc(Oc2ccc(F)cc2)ccn1 |r| Show InChI InChI=1S/C34H40ClFN10O4/c1-20(2)17-29(31(47)41-19-24-18-27(14-16-39-24)50-26-12-8-23(36)9-13-26)44-32(48)28(5-4-15-40-34(37)38)43-33(49)30-42-21(3)46(45-30)25-10-6-22(35)7-11-25/h6-14,16,18,20,28-29H,4-5,15,17,19H2,1-3H3,(H,41,47)(H,43,49)(H,44,48)(H4,37,38,40)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182652
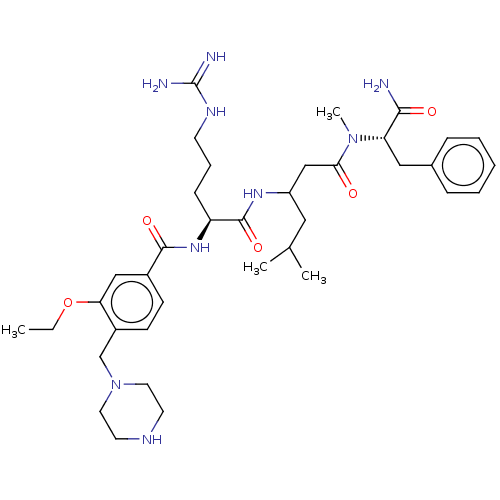
(CHEMBL3818255)Show SMILES CCOc1cc(ccc1CN1CCNCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H57N9O5/c1-5-51-32-22-27(13-14-28(32)24-46-18-16-41-17-19-46)35(49)44-30(12-9-15-42-37(39)40)36(50)43-29(20-25(2)3)23-33(47)45(4)31(34(38)48)21-26-10-7-6-8-11-26/h6-8,10-11,13-14,22,25,29-31,41H,5,9,12,15-21,23-24H2,1-4H3,(H2,38,48)(H,43,50)(H,44,49)(H4,39,40,42)/t29?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490473
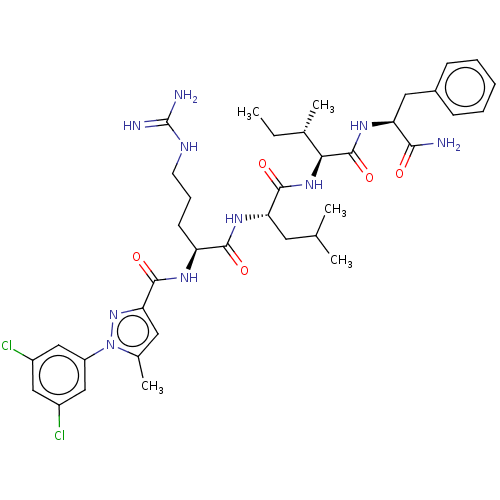
(CHEMBL2326557)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(C)n(n1)-c1cc(Cl)cc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C38H52Cl2N10O5/c1-6-22(4)32(37(55)46-29(33(41)51)17-24-11-8-7-9-12-24)48-35(53)30(15-21(2)3)47-34(52)28(13-10-14-44-38(42)43)45-36(54)31-16-23(5)50(49-31)27-19-25(39)18-26(40)20-27/h7-9,11-12,16,18-22,28-30,32H,6,10,13-15,17H2,1-5H3,(H2,41,51)(H,45,54)(H,46,55)(H,47,52)(H,48,53)(H4,42,43,44)/t22-,28-,29-,30-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50444664

(CHEMBL3098658)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-c1ccc(cn1)-[#7]-1-[#6]-[#6]-[#7]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C37H56N10O6/c1-5-24(4)31(35(51)45-30(36(52)53)21-25-10-7-6-8-11-25)46-34(50)29(20-23(2)3)44-33(49)28(12-9-15-41-37(38)39)43-32(48)27-14-13-26(22-42-27)47-18-16-40-17-19-47/h6-8,10-11,13-14,22-24,28-31,40H,5,9,12,15-21H2,1-4H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H,52,53)(H4,38,39,41)/t24-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK4/cyclin D1 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate after 45 mins by fluorescenc... |
Bioorg Med Chem 22: 616-22 (2013)
Article DOI: 10.1016/j.bmc.2013.10.039
BindingDB Entry DOI: 10.7270/Q2416ZHS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182725
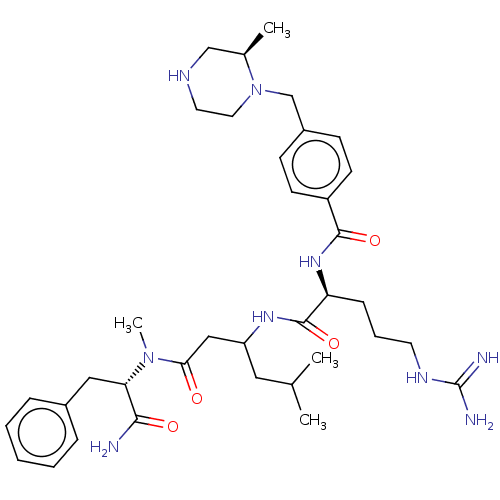
(CHEMBL3818786)Show SMILES CC(C)CC(CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CN2CCNC[C@H]2C)cc1 |r| Show InChI InChI=1S/C36H55N9O4/c1-24(2)19-29(21-32(46)44(4)31(33(37)47)20-26-9-6-5-7-10-26)42-35(49)30(11-8-16-41-36(38)39)43-34(48)28-14-12-27(13-15-28)23-45-18-17-40-22-25(45)3/h5-7,9-10,12-15,24-25,29-31,40H,8,11,16-23H2,1-4H3,(H2,37,47)(H,42,49)(H,43,48)(H4,38,39,41)/t25-,29?,30+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182720

(CHEMBL3819344)Show SMILES CCCN1CCN(Cc2ccc(cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)NC(Cc2ccsc2)C(N)=O)[C@@H](C)C1 |r| Show InChI InChI=1S/C36H57N9O4S/c1-5-14-44-15-16-45(25(4)21-44)22-26-8-10-28(11-9-26)34(48)43-30(7-6-13-40-36(38)39)35(49)41-29(18-24(2)3)20-32(46)42-31(33(37)47)19-27-12-17-50-23-27/h8-12,17,23-25,29-31H,5-7,13-16,18-22H2,1-4H3,(H2,37,47)(H,41,49)(H,42,46)(H,43,48)(H4,38,39,40)/t25-,29?,30-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182649

(CHEMBL3817868)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(OC2CCNCC2)c(OC)c1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H60N8O8/c1-6-25(4)34(38(52)47-31(39(53)54)22-26-11-8-7-9-12-26)48-37(51)30(21-24(2)3)46-36(50)29(13-10-18-44-40(41)42)45-35(49)27-14-15-32(33(23-27)55-5)56-28-16-19-43-20-17-28/h7-9,11-12,14-15,23-25,28-31,34,43H,6,10,13,16-22H2,1-5H3,(H,45,49)(H,46,50)(H,47,52)(H,48,51)(H,53,54)(H4,41,42,44)/t25-,29-,30-,31-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182652

(CHEMBL3818255)Show SMILES CCOc1cc(ccc1CN1CCNCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)C)CC(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H57N9O5/c1-5-51-32-22-27(13-14-28(32)24-46-18-16-41-17-19-46)35(49)44-30(12-9-15-42-37(39)40)36(50)43-29(20-25(2)3)23-33(47)45(4)31(34(38)48)21-26-10-7-6-8-11-26/h6-8,10-11,13-14,22,25,29-31,41H,5,9,12,15-21,23-24H2,1-4H3,(H2,38,48)(H,43,50)(H,44,49)(H4,39,40,42)/t29?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50182729

(CHEMBL3818382)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(cc1)N1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C38H57N9O6/c1-5-25(4)32(36(51)45-31(37(52)53)23-26-10-7-6-8-11-26)46-35(50)30(22-24(2)3)44-34(49)29(12-9-17-42-38(39)40)43-33(48)27-13-15-28(16-14-27)47-20-18-41-19-21-47/h6-8,10-11,13-16,24-25,29-32,41H,5,9,12,17-23H2,1-4H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H,52,53)(H4,39,40,42)/t25-,29-,30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK4/cyclin D1 using fluoresceinyl-AhxPro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly as substrate incubated for 45 mins by fluo... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182704
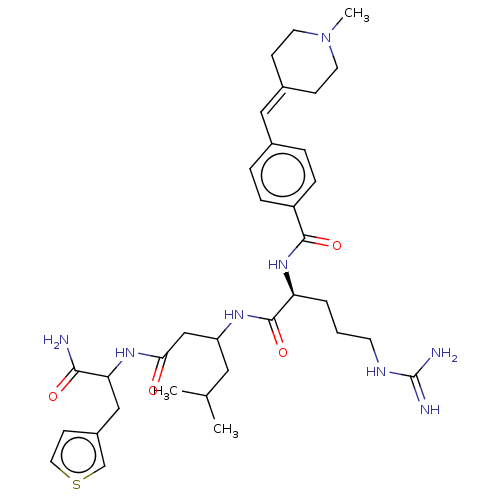
(CHEMBL3818715)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6](-[#6]-[#6](=O)-[#7]-[#6](-[#6]-c1ccsc1)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-c1ccc(\[#6]=[#6]-2/[#6]-[#6]-[#7](-[#6])-[#6]-[#6]-2)cc1 |r| Show InChI InChI=1S/C34H50N8O4S/c1-22(2)17-27(20-30(43)40-29(31(35)44)19-25-12-16-47-21-25)39-33(46)28(5-4-13-38-34(36)37)41-32(45)26-8-6-23(7-9-26)18-24-10-14-42(3)15-11-24/h6-9,12,16,18,21-22,27-29H,4-5,10-11,13-15,17,19-20H2,1-3H3,(H2,35,44)(H,39,46)(H,40,43)(H,41,45)(H4,36,37,38)/t27?,28-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50490482

(CHEMBL2326554)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1cccc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H52ClN11O5/c1-6-22(4)30(35(53)45-28(31(39)50)19-24-12-8-7-9-13-24)47-34(52)29(18-21(2)3)46-33(51)27(16-11-17-42-37(40)41)44-36(54)32-43-23(5)49(48-32)26-15-10-14-25(38)20-26/h7-10,12-15,20-22,27-30H,6,11,16-19H2,1-5H3,(H2,39,50)(H,44,54)(H,45,53)(H,46,51)(H,47,52)(H4,40,41,42)/t22-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK4/Cyclin D1 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50490475

(CHEMBL2326552)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1nc(C)n(n1)-c1cc(Cl)cc(Cl)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H51Cl2N11O5/c1-6-21(4)30(35(54)46-28(31(40)51)16-23-11-8-7-9-12-23)48-34(53)29(15-20(2)3)47-33(52)27(13-10-14-43-37(41)42)45-36(55)32-44-22(5)50(49-32)26-18-24(38)17-25(39)19-26/h7-9,11-12,17-21,27-30H,6,10,13-16H2,1-5H3,(H2,40,51)(H,45,55)(H,46,54)(H,47,52)(H,48,53)(H4,41,42,43)/t21-,27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK4/Cyclin D1 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444673

(CHEMBL3098654)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-c1ccccn1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(F)cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C31H42FN9O7/c1-17(2)14-22(39-27(44)21(7-5-13-37-31(34)35)38-26(43)20-6-3-4-12-36-20)28(45)40-23(16-25(33)42)29(46)41-24(30(47)48)15-18-8-10-19(32)11-9-18/h3-4,6,8-12,17,21-24H,5,7,13-16H2,1-2H3,(H2,33,42)(H,38,43)(H,39,44)(H,40,45)(H,41,46)(H,47,48)(H4,34,35,37)/t21-,22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/cyclin A using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate after 45 mins by fluorescence pola... |
Bioorg Med Chem 22: 616-22 (2013)
Article DOI: 10.1016/j.bmc.2013.10.039
BindingDB Entry DOI: 10.7270/Q2416ZHS |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50490480
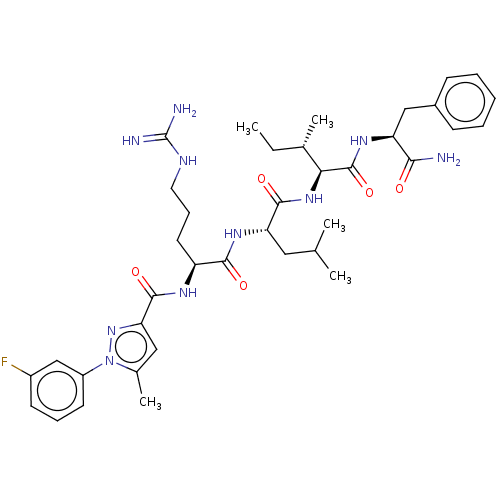
(CHEMBL2326559)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(C)n(n1)-c1cccc(F)c1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C38H53FN10O5/c1-6-23(4)32(37(54)45-29(33(40)50)20-25-12-8-7-9-13-25)47-35(52)30(18-22(2)3)46-34(51)28(16-11-17-43-38(41)42)44-36(53)31-19-24(5)49(48-31)27-15-10-14-26(39)21-27/h7-10,12-15,19,21-23,28-30,32H,6,11,16-18,20H2,1-5H3,(H2,40,50)(H,44,53)(H,45,54)(H,46,51)(H,47,52)(H4,41,42,43)/t23-,28-,29-,30-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK2/Cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide as substrate after 45 mins by f... |
J Med Chem 56: 1573-82 (2013)
Article DOI: 10.1021/jm3013882
BindingDB Entry DOI: 10.7270/Q2QV3QFD |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182733

(CHEMBL3819004)Show SMILES CC(C)CC(CC(=O)NC(Cc1ccsc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccc(CC2CCNCC2)cc1 |r| Show InChI InChI=1S/C33H50N8O4S/c1-21(2)16-26(19-29(42)40-28(30(34)43)18-24-11-15-46-20-24)39-32(45)27(4-3-12-38-33(35)36)41-31(44)25-7-5-22(6-8-25)17-23-9-13-37-14-10-23/h5-8,11,15,20-21,23,26-28,37H,3-4,9-10,12-14,16-19H2,1-2H3,(H2,34,43)(H,39,45)(H,40,42)(H,41,44)(H4,35,36,38)/t26?,27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50182738
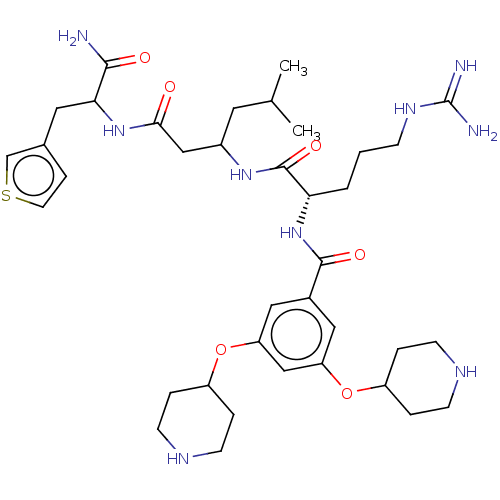
(CHEMBL3818052)Show SMILES CC(C)CC(CC(=O)NC(Cc1ccsc1)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(OC2CCNCC2)cc(OC2CCNCC2)c1 |r| Show InChI InChI=1S/C37H57N9O6S/c1-23(2)16-26(20-33(47)45-32(34(38)48)17-24-9-15-53-22-24)44-36(50)31(4-3-10-43-37(39)40)46-35(49)25-18-29(51-27-5-11-41-12-6-27)21-30(19-25)52-28-7-13-42-14-8-28/h9,15,18-19,21-23,26-28,31-32,41-42H,3-8,10-14,16-17,20H2,1-2H3,(H2,38,48)(H,44,50)(H,45,47)(H,46,49)(H4,39,40,43)/t26?,31-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK2/cyclin A2 using fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly as substrate incubated for 45 mins by fluoresc... |
Bioorg Med Chem Lett 26: 3754-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.067
BindingDB Entry DOI: 10.7270/Q21Z46B2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data