

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035236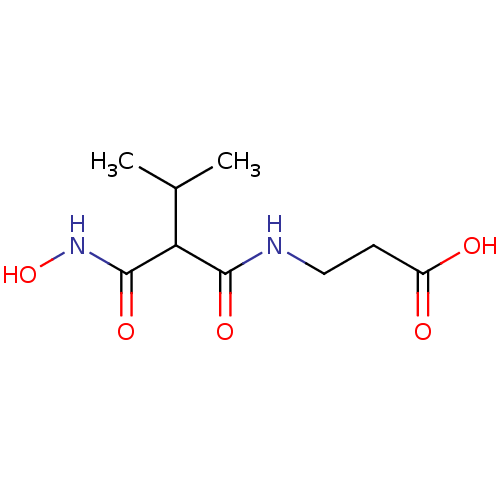 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035247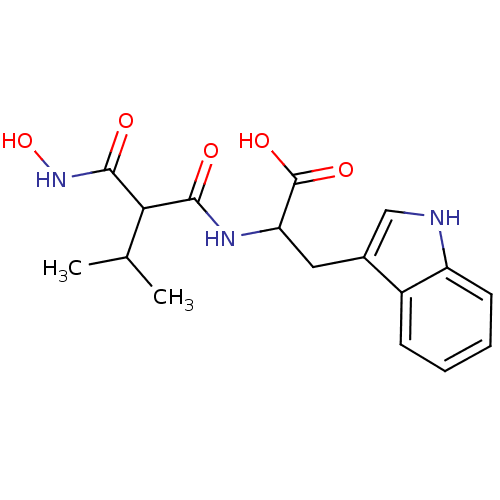 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229504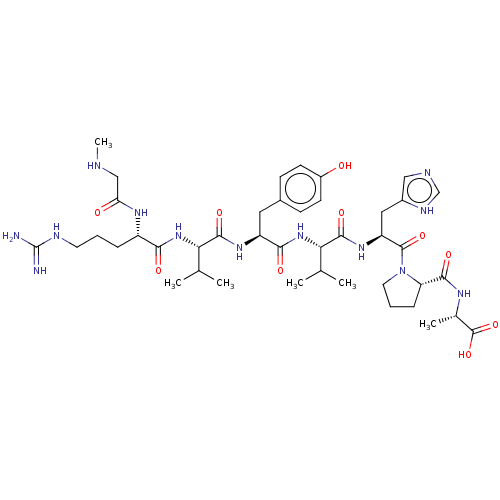 (Saralasin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity as antagonist of AII on guinea pig ileum | J Med Chem 34: 2402-10 (1991) BindingDB Entry DOI: 10.7270/Q2VD71PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035247 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030815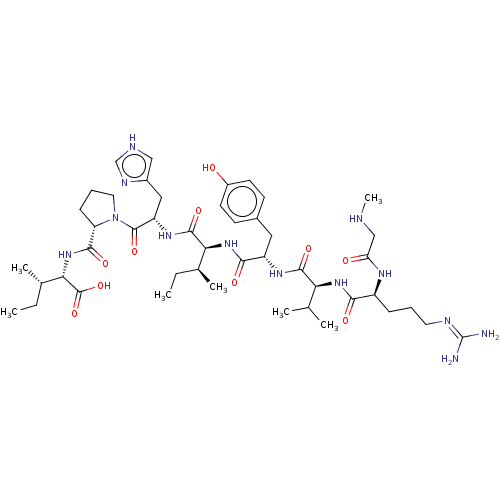 (CHEMBL404594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity as antagonist of AII on guinea pig ileum | J Med Chem 34: 2402-10 (1991) BindingDB Entry DOI: 10.7270/Q2VD71PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035247 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229420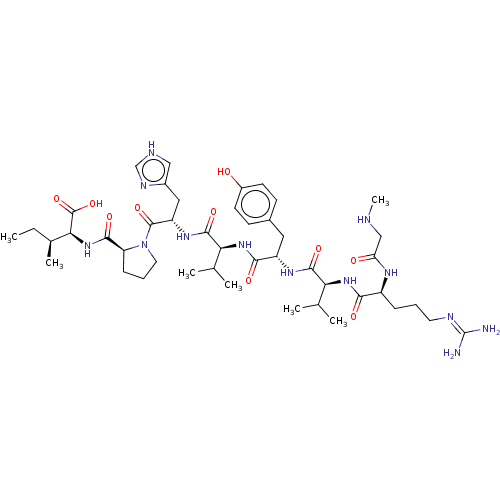 (CHEMBL77838) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity as antagonist of AII on guinea pig ileum | J Med Chem 34: 2402-10 (1991) BindingDB Entry DOI: 10.7270/Q2VD71PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035236 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50105264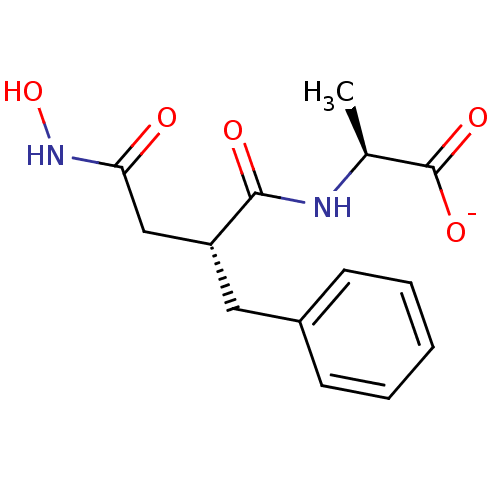 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035251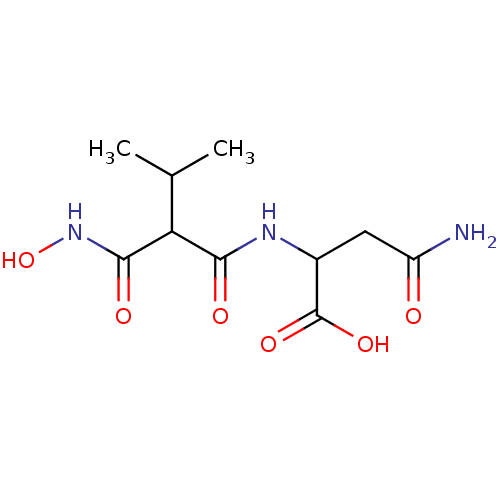 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-succi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035239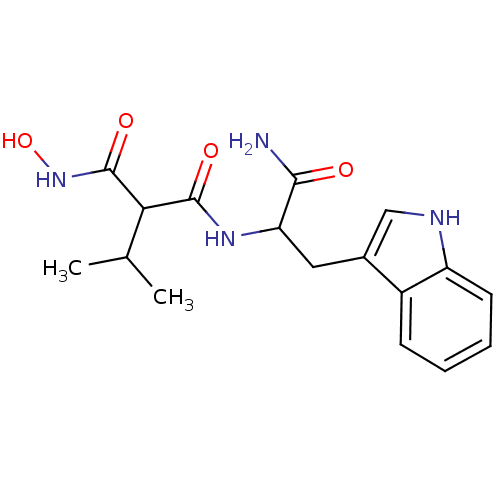 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035236 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035239 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035235 (CHEMBL2372437 | N-[1-(Carbamoylmethyl-carbamoyl)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50105264 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035251 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-succi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035240 ((2-Hydroxycarbamoyl-3-methyl-butyrylamino)-acetic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035239 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035237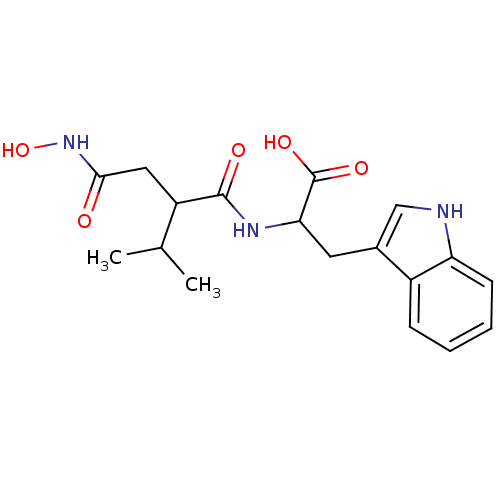 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21641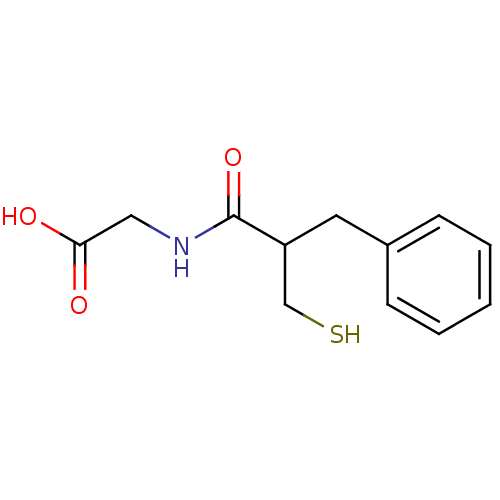 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035247 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50228894 (CHEMBL312754) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity as antagonist of AII on guinea pig ileum | J Med Chem 34: 2402-10 (1991) BindingDB Entry DOI: 10.7270/Q2VD71PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035240 ((2-Hydroxycarbamoyl-3-methyl-butyrylamino)-acetic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229419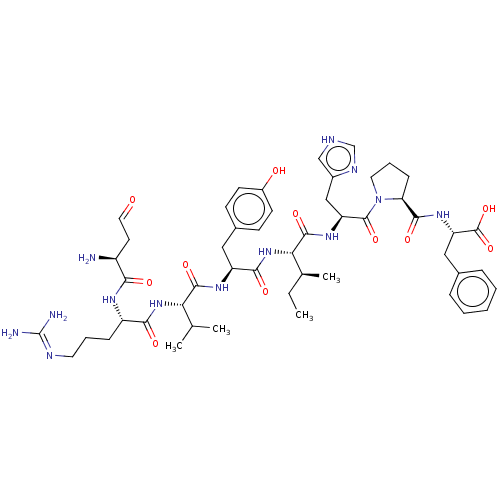 (CHEMBL405381) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to displace [3H]Angiotensin II (300 ng/mL) from rabbit adrenal cortex | J Med Chem 34: 2402-10 (1991) BindingDB Entry DOI: 10.7270/Q2VD71PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50105264 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035240 ((2-Hydroxycarbamoyl-3-methyl-butyrylamino)-acetic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50105264 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035239 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035235 (CHEMBL2372437 | N-[1-(Carbamoylmethyl-carbamoyl)-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035237 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035237 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035251 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-succi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035237 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035250 (3-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035235 (CHEMBL2372437 | N-[1-(Carbamoylmethyl-carbamoyl)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035243 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 262 total ) | Next | Last >> |