

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50468724 (CHEMBL4294334) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50468723 (CHEMBL4279824) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Falcipain 2 (Plasmodium falciparum) | BDBM50183767 ((2R,3R)-dibenzyl 1-((S)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain 2 using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50183767 ((2R,3R)-dibenzyl 1-((S)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of human cathepsin-L using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Falcipain 2 (Plasmodium falciparum) | BDBM50468724 (CHEMBL4294334) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain 2 using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50183767 ((2R,3R)-dibenzyl 1-((S)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50468724 (CHEMBL4294334) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50183757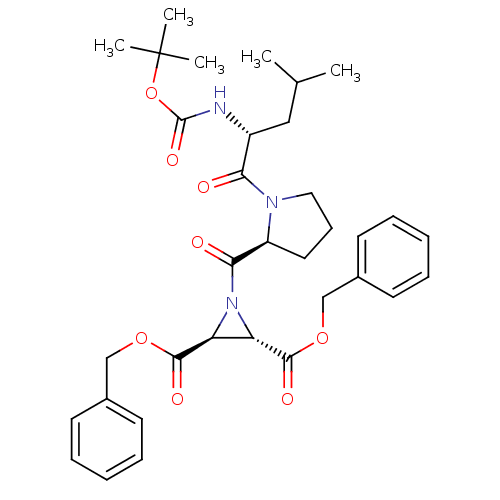 ((2S,3S)-dibenzyl 1-((S)-1-((R)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50183776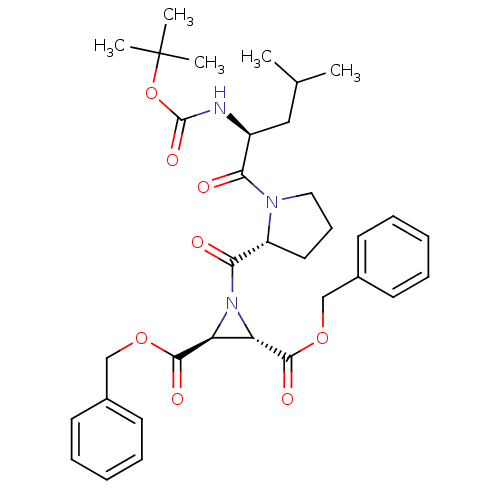 ((2S,3S)-dibenzyl 1-((R)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50183757 ((2S,3S)-dibenzyl 1-((S)-1-((R)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50183776 ((2S,3S)-dibenzyl 1-((R)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Falcipain 2 (Plasmodium falciparum) | BDBM50468723 (CHEMBL4279824) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain 2 using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50468729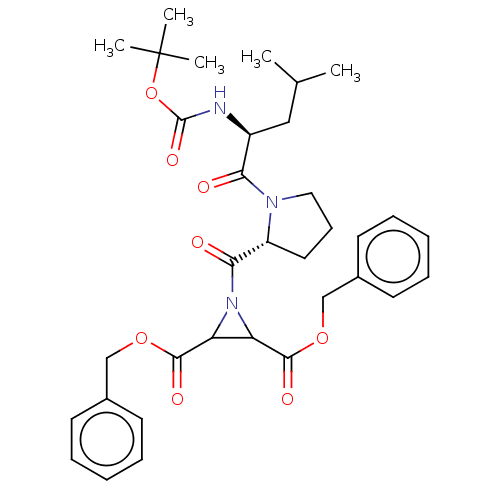 (CHEMBL4288606) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50468725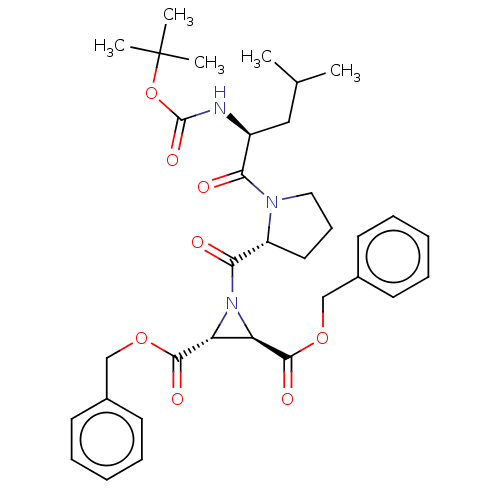 (CHEMBL4279254) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50468729 (CHEMBL4288606) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50183762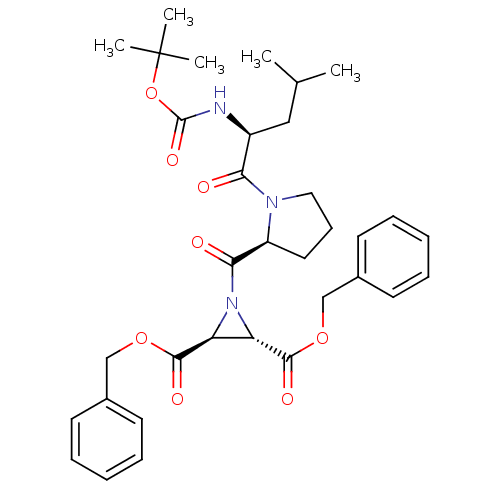 ((2S,3S)-dibenzyl 1-((S)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50183747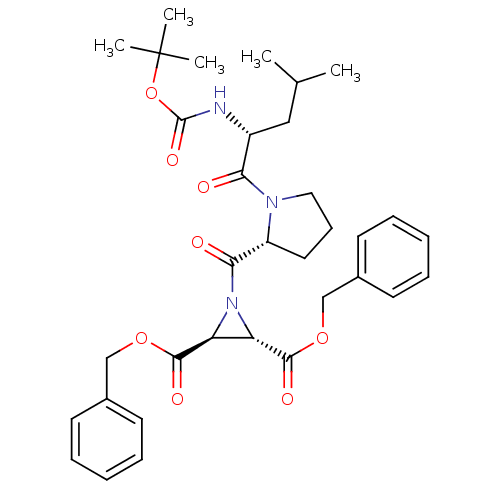 ((2S,3S)-dibenzyl 1-((R)-1-((R)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50183757 ((2S,3S)-dibenzyl 1-((S)-1-((R)-2-(tert-butoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of human cathepsin-L using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50468722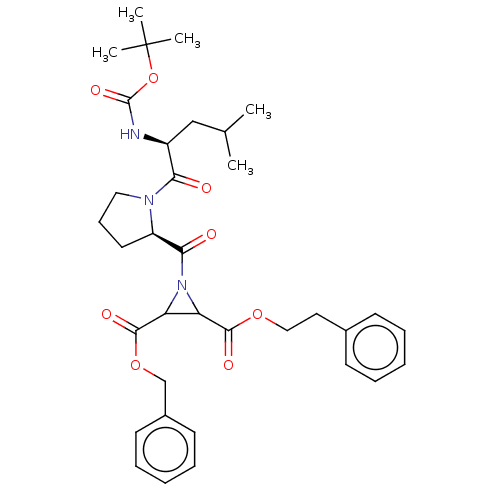 (CHEMBL4292021) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50183776 ((2S,3S)-dibenzyl 1-((R)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of human cathepsin-L using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50468724 (CHEMBL4294334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of human cathepsin-L using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50468726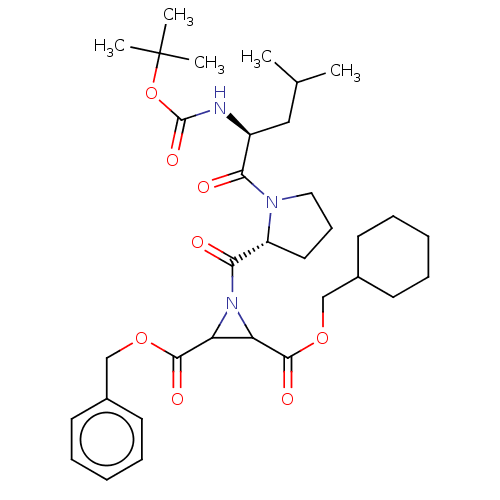 (CHEMBL4283566) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50468727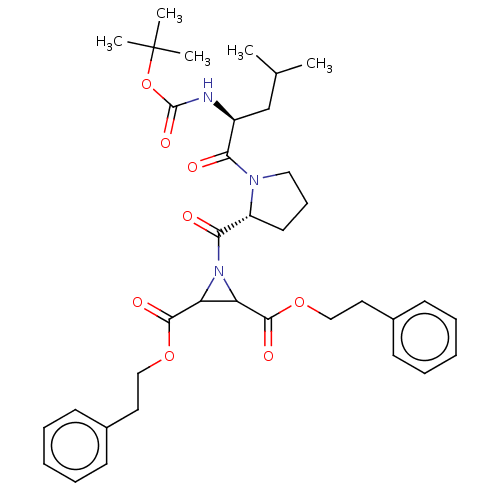 (CHEMBL4286203) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50468729 (CHEMBL4288606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of human cathepsin-L using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50468721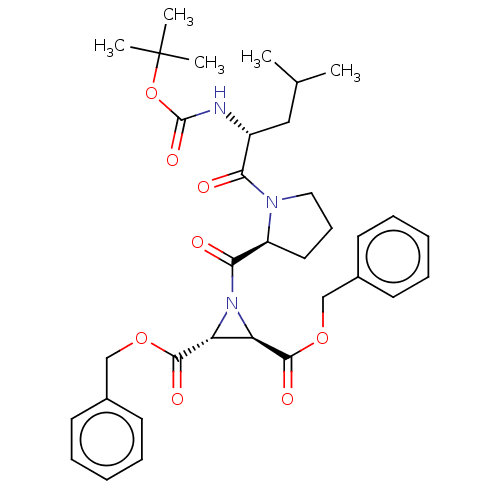 (CHEMBL4289948) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B-like protease (Leishmania major) | BDBM50468725 (CHEMBL4279254) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Leishmania major MHOM/IL/81/FE/BNI His6-tagged CPC expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate by fluorescence spec... | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Falcipain 2 (Plasmodium falciparum) | BDBM50183757 ((2S,3S)-dibenzyl 1-((S)-1-((R)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain 2 using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Falcipain 2 (Plasmodium falciparum) | BDBM50468728 (CHEMBL4290479) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain 2 using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50468721 (CHEMBL4289948) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Falcipain 2 (Plasmodium falciparum) | BDBM50183776 ((2S,3S)-dibenzyl 1-((R)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum falcipain 2 using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50183767 ((2R,3R)-dibenzyl 1-((S)-1-((S)-2-(tert-butoxycarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-Universit£t Mainz Curated by ChEMBL | Assay Description Inhibition of human cathepsin-B using Z-Phe-Arg-AMC as substrate by fluorometric assay | Eur J Med Chem 156: 587-597 (2018) Article DOI: 10.1016/j.ejmech.2018.07.012 BindingDB Entry DOI: 10.7270/Q2CN76MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||