 Found 178 hits with Last Name = 'maier' and Initial = 'r'
Found 178 hits with Last Name = 'maier' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615937
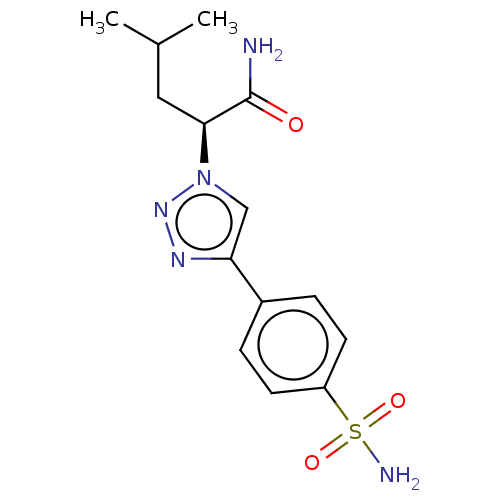
(CHEMBL5284282)Show SMILES CC(C)C[C@@H](C(N)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615941

(CHEMBL5290825)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615937

(CHEMBL5284282)Show SMILES CC(C)C[C@@H](C(N)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615934

(CHEMBL5266613)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615943
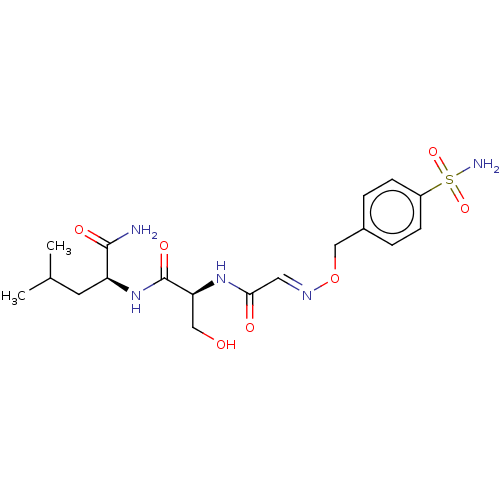
(CHEMBL5282667)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615938

(CHEMBL5281859)Show SMILES C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615942

(CHEMBL5271039)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615944

(CHEMBL5274201)Show SMILES NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615939
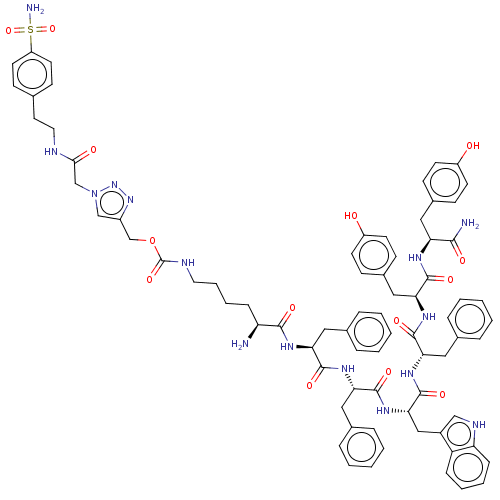
(CHEMBL5280346)Show SMILES N[C@@H](CCCCNC(=O)OCc1cn(CC(=O)NCCc2ccc(cc2)S(N)(=O)=O)nn1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50236140
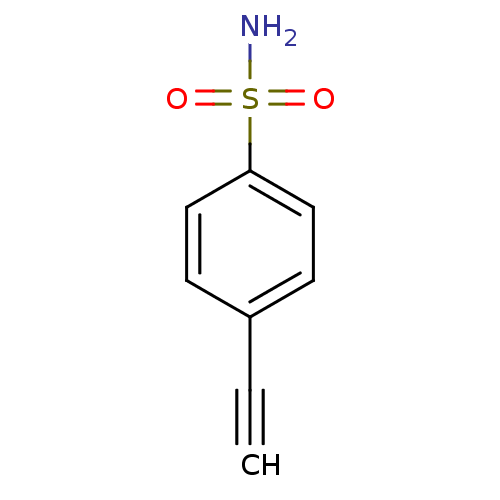
(4-ethynyl benzene sulfonamide | 4-ethynylbenzenesu...)Show InChI InChI=1S/C8H7NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h1,3-6H,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615940

(CHEMBL5275727)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCCCNC(=O)OCc1cn(CC(=O)NCCc2ccc(cc2)S(N)(=O)=O)nn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615940

(CHEMBL5275727)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCCCNC(=O)OCc1cn(CC(=O)NCCc2ccc(cc2)S(N)(=O)=O)nn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615941

(CHEMBL5290825)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615939

(CHEMBL5280346)Show SMILES N[C@@H](CCCCNC(=O)OCc1cn(CC(=O)NCCc2ccc(cc2)S(N)(=O)=O)nn1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615934

(CHEMBL5266613)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50236140

(4-ethynyl benzene sulfonamide | 4-ethynylbenzenesu...)Show InChI InChI=1S/C8H7NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h1,3-6H,(H2,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615943

(CHEMBL5282667)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615942

(CHEMBL5271039)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615944

(CHEMBL5274201)Show SMILES NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615938

(CHEMBL5281859)Show SMILES C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615935

(CHEMBL5287147) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 471 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50615936

(CHEMBL5278617) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 485 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50615935

(CHEMBL5287147) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 601 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615935

(CHEMBL5287147) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 844 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615941

(CHEMBL5290825)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 936 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615936

(CHEMBL5278617) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615934

(CHEMBL5266613)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615944

(CHEMBL5274201)Show SMILES NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615943

(CHEMBL5282667)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50615942

(CHEMBL5271039)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50615944

(CHEMBL5274201)Show SMILES NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50615935

(CHEMBL5287147) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50615942

(CHEMBL5271039)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50615943

(CHEMBL5282667)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50615934

(CHEMBL5266613)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50615941

(CHEMBL5290825)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)\C=N\OCc1ccc(cc1)S(N)(=O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224600
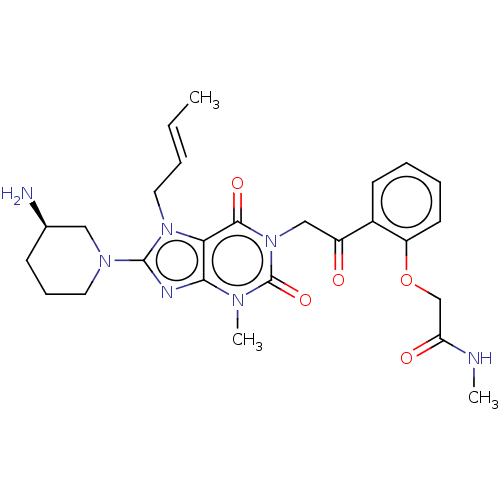
(US10202383, Example 2(148) | US9321791, 2(148) | U...)Show SMILES CNC(=O)COc1ccccc1C(=O)Cn1c(=O)n(C)c2nc(N3CCC[C@@H](N)C3)n(C\C=C\C)c2c1=O Show InChI InChI=1S/C26H33N7O5/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-17(27)14-31)30(3)26(37)33(24(22)36)15-19(34)18-10-6-7-11-20(18)38-16-21(35)28-2/h4-7,10-11,17H,8-9,12-16,27H2,1-3H3,(H,28,35)/b5-4+/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US10202383 (2019)
BindingDB Entry DOI: 10.7270/Q2P55QNN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228394
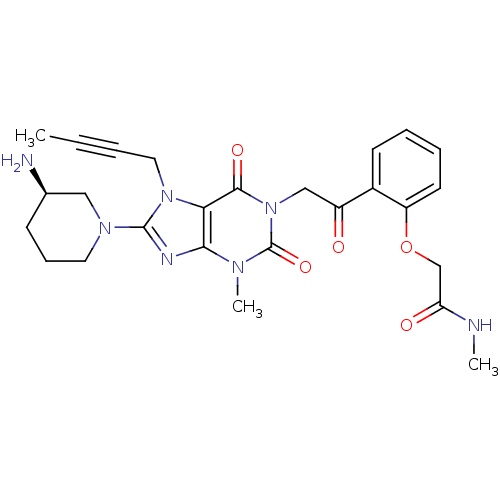
((R)-2-(2-(2-(8-(3-aminopiperidin-1-yl)-7-(but-2-yn...)Show SMILES CNC(=O)COc1ccccc1C(=O)Cn1c(=O)n(C)c2nc(N3CCC[C@@H](N)C3)n(CC#CC)c2c1=O Show InChI InChI=1S/C26H31N7O5/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-17(27)14-31)30(3)26(37)33(24(22)36)15-19(34)18-10-6-7-11-20(18)38-16-21(35)28-2/h6-7,10-11,17H,8-9,12-16,27H2,1-3H3,(H,28,35)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US9556175 (2017)
BindingDB Entry DOI: 10.7270/Q2Z03B5T |
More data for this
Ligand-Target Pair | |
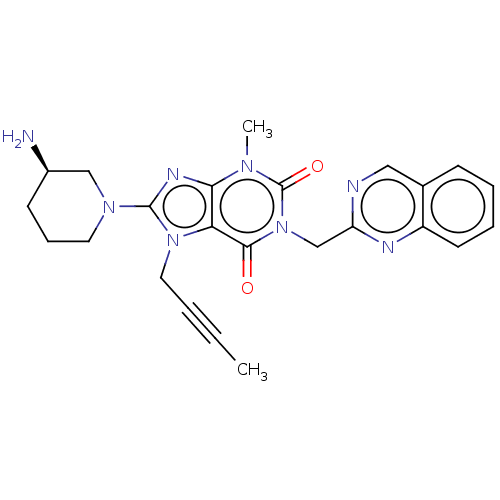
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224587
(US10202383, Example 2(80) | US9321791, 2(80) | US9...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3ncc4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C24H26N8O2/c1-3-4-12-31-20-21(28-23(31)30-11-7-9-17(25)14-30)29(2)24(34)32(22(20)33)15-19-26-13-16-8-5-6-10-18(16)27-19/h5-6,8,10,13,17H,7,9,11-12,14-15,25H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US10202383 (2019)
BindingDB Entry DOI: 10.7270/Q2P55QNN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224587

(US10202383, Example 2(80) | US9321791, 2(80) | US9...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3ncc4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C24H26N8O2/c1-3-4-12-31-20-21(28-23(31)30-11-7-9-17(25)14-30)29(2)24(34)32(22(20)33)15-19-26-13-16-8-5-6-10-18(16)27-19/h5-6,8,10,13,17H,7,9,11-12,14-15,25H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... |
US Patent US9321791 (2016)
BindingDB Entry DOI: 10.7270/Q2GQ6WNR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224601
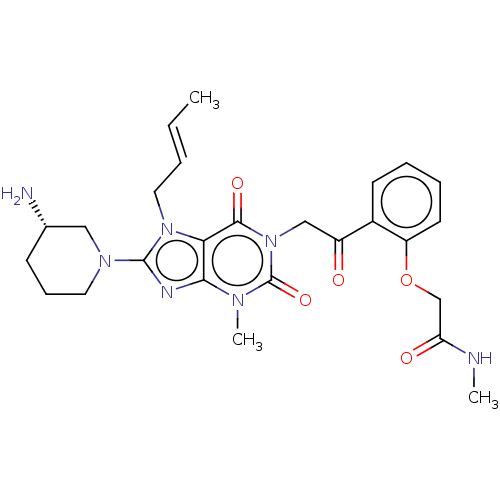
(US10202383, Example 2(150) | US9321791, 2(150) | U...)Show SMILES CNC(=O)COc1ccccc1C(=O)Cn1c(=O)n(C)c2nc(N3CCC[C@H](N)C3)n(C\C=C\C)c2c1=O Show InChI InChI=1S/C26H33N7O5/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-17(27)14-31)30(3)26(37)33(24(22)36)15-19(34)18-10-6-7-11-20(18)38-16-21(35)28-2/h4-7,10-11,17H,8-9,12-16,27H2,1-3H3,(H,28,35)/b5-4+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US9556175 (2017)
BindingDB Entry DOI: 10.7270/Q2Z03B5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224600

(US10202383, Example 2(148) | US9321791, 2(148) | U...)Show SMILES CNC(=O)COc1ccccc1C(=O)Cn1c(=O)n(C)c2nc(N3CCC[C@@H](N)C3)n(C\C=C\C)c2c1=O Show InChI InChI=1S/C26H33N7O5/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-17(27)14-31)30(3)26(37)33(24(22)36)15-19(34)18-10-6-7-11-20(18)38-16-21(35)28-2/h4-7,10-11,17H,8-9,12-16,27H2,1-3H3,(H,28,35)/b5-4+/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US9556175 (2017)
BindingDB Entry DOI: 10.7270/Q2Z03B5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US9556175 (2017)
BindingDB Entry DOI: 10.7270/Q2Z03B5T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224594
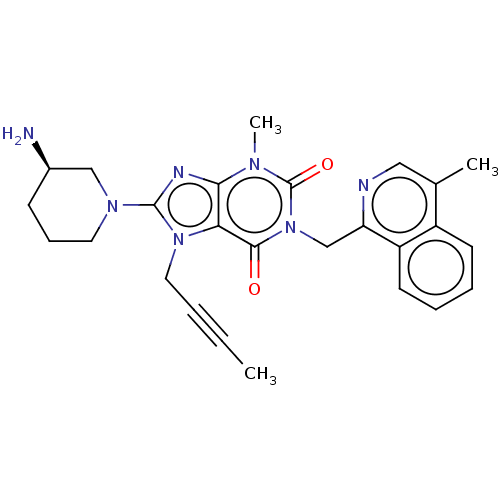
(US10202383, Example 2(132) | US9321791, 2(132) | U...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3ncc(C)c4ccccc34)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C26H29N7O2/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-18(27)15-31)30(3)26(35)33(24(22)34)16-21-20-11-7-6-10-19(20)17(2)14-28-21/h6-7,10-11,14,18H,8-9,12-13,15-16,27H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates.... |
US Patent US9556175 (2017)
BindingDB Entry DOI: 10.7270/Q2Z03B5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228394

((R)-2-(2-(2-(8-(3-aminopiperidin-1-yl)-7-(but-2-yn...)Show SMILES CNC(=O)COc1ccccc1C(=O)Cn1c(=O)n(C)c2nc(N3CCC[C@@H](N)C3)n(CC#CC)c2c1=O Show InChI InChI=1S/C26H31N7O5/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-17(27)14-31)30(3)26(37)33(24(22)36)15-19(34)18-10-6-7-11-20(18)38-16-21(35)28-2/h6-7,10-11,17H,8-9,12-16,27H2,1-3H3,(H,28,35)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... |
US Patent US9321791 (2016)
BindingDB Entry DOI: 10.7270/Q2GQ6WNR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM224594

(US10202383, Example 2(132) | US9321791, 2(132) | U...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3ncc(C)c4ccccc34)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C26H29N7O2/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-18(27)15-31)30(3)26(35)33(24(22)34)16-21-20-11-7-6-10-19(20)17(2)14-28-21/h6-7,10-11,14,18H,8-9,12-13,15-16,27H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, were placed in black microtitre plates. 20 ... |
US Patent US9321791 (2016)
BindingDB Entry DOI: 10.7270/Q2GQ6WNR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data