

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50332805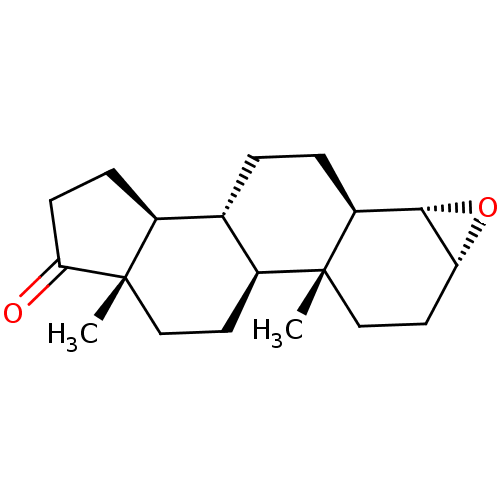 ((3R,4S,5R,8R,9S,10R,13S,14S)-10,13-Dimethyl-hexade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332803 ((5S,8R,9S,10S,13S,14S)-10,13-dimethyl-5,6,7,8,9,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388393 (CHEMBL2058266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388396 (CHEMBL1077603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388394 (CHEMBL2058267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50240798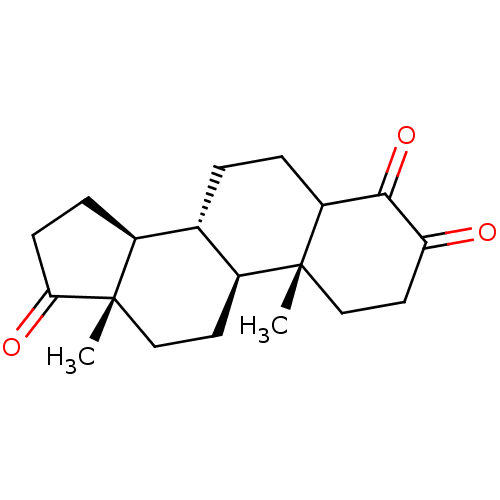 ((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50398447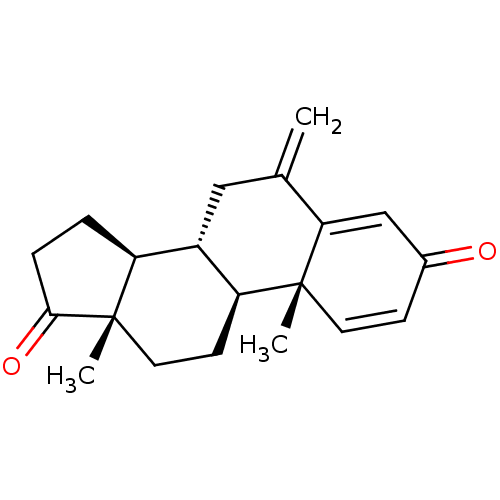 (Aromasin | EXEMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069753 (CHEMBL3407538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50168058 (CHEMBL3798461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332807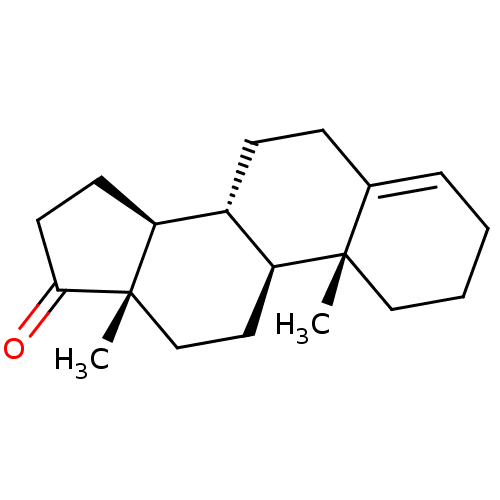 ((8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,7,8,9,10,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332805 ((3R,4S,5R,8R,9S,10R,13S,14S)-10,13-Dimethyl-hexade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388396 (CHEMBL1077603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332803 ((5S,8R,9S,10S,13S,14S)-10,13-dimethyl-5,6,7,8,9,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069753 (CHEMBL3407538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50168056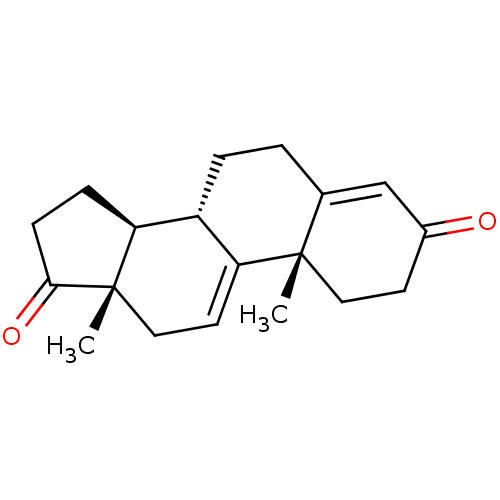 (CHEMBL3799363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50123151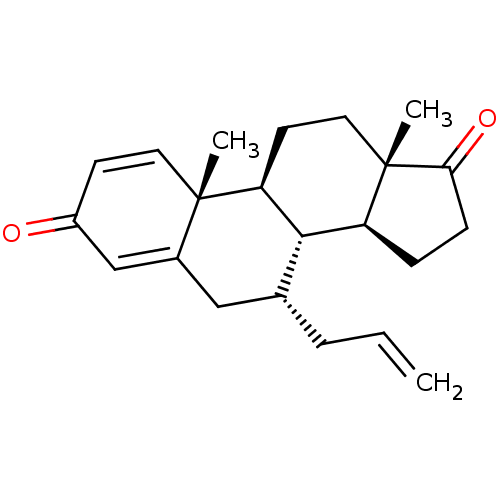 (CHEMBL3623223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50168057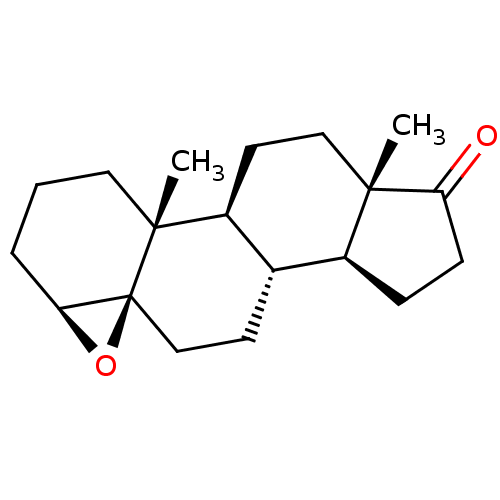 (CHEMBL3800417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069755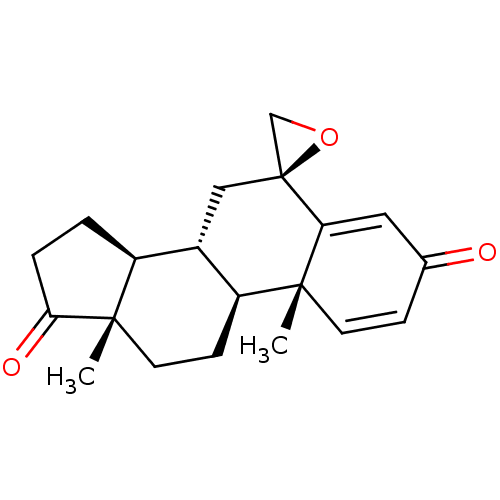 (CHEMBL3407536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069752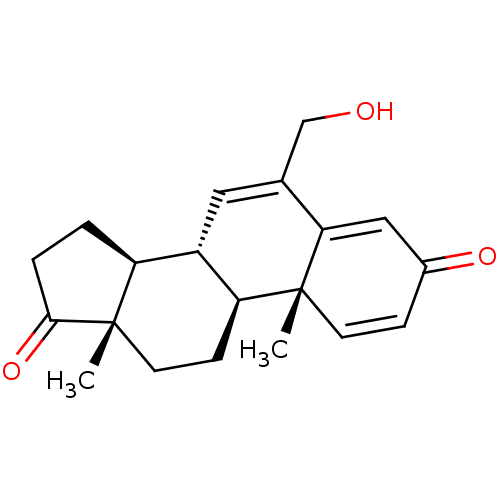 (CHEMBL3407539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069755 (CHEMBL3407536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069754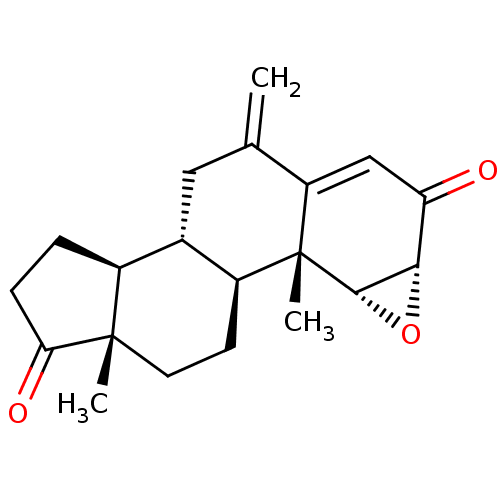 (CHEMBL3407537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50398447 (Aromasin | EXEMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388393 (CHEMBL2058266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069752 (CHEMBL3407539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388395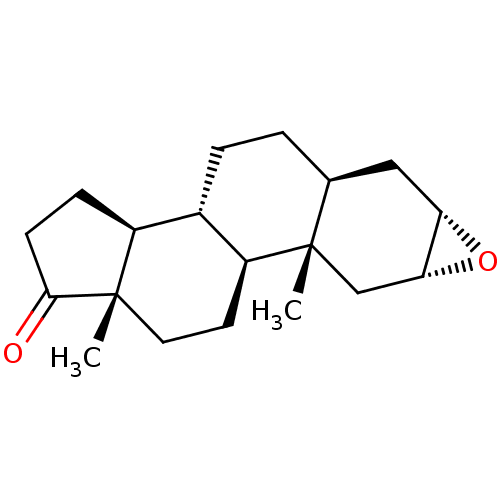 (CHEMBL2058268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069754 (CHEMBL3407537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50388394 (CHEMBL2058267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]androstenedione as substrate after 15 mins by liquid scintillation counting | J Med Chem 55: 3992-4002 (2012) Article DOI: 10.1021/jm300262w BindingDB Entry DOI: 10.7270/Q22V2H52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||