

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025238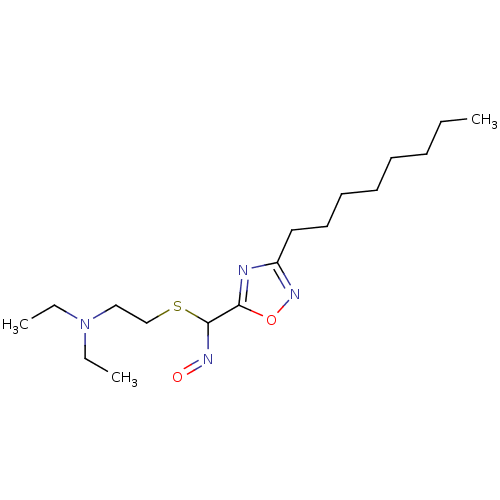 (CHEMBL71740 | N-Hydroxy-3-octyl-[1,2,4]oxadiazole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the concentration required for reversible inhibition of human acetylcholinesterase . | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025251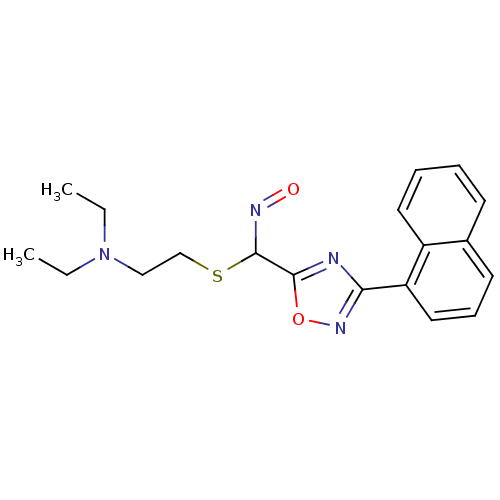 (CHEMBL302946 | N-Hydroxy-3-naphthalen-1-yl-[1,2,4]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025238 (CHEMBL71740 | N-Hydroxy-3-octyl-[1,2,4]oxadiazole-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the concentration required for reversible inhibition of eel acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025242 (CHEMBL20339 | CHEMBL2079594 | N-Hydroxy-3-phenyl-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025242 (CHEMBL20339 | CHEMBL2079594 | N-Hydroxy-3-phenyl-[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025239 (3-Benzyl-N-hydroxy-[1,2,4]oxadiazole-5-carboximido...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025239 (3-Benzyl-N-hydroxy-[1,2,4]oxadiazole-5-carboximido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025247 (3-tert-Butyl-N-hydroxy-[1,2,4]oxadiazole-5-carboxi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025247 (3-tert-Butyl-N-hydroxy-[1,2,4]oxadiazole-5-carboxi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025252 (CHEMBL19557 | N-Hydroxy-3-methyl-[1,2,4]oxadiazole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025252 (CHEMBL19557 | N-Hydroxy-3-methyl-[1,2,4]oxadiazole...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025242 (CHEMBL20339 | CHEMBL2079594 | N-Hydroxy-3-phenyl-[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025253 (CHEMBL69059 | N-(2-Diethylamino-ethyl)-N'-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020514 (2-(Hydroxyimino-methyl)-1-methoxymethyl-3-methyl-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the HOX that inhibits 50% of AChE (Acetylcholinesterase) activity | J Med Chem 27: 1431-8 (1984) BindingDB Entry DOI: 10.7270/Q2TH8KP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025241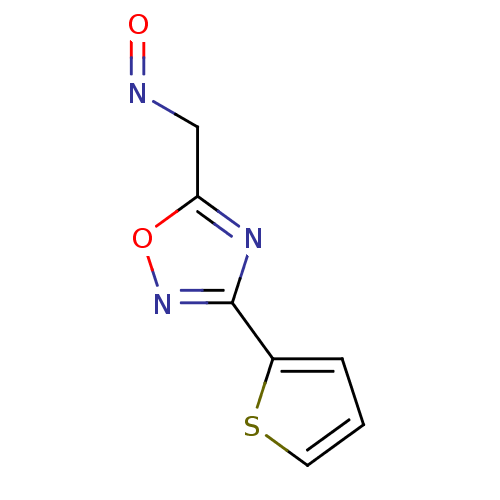 (3-Thiophen-2-yl-[1,2,4]oxadiazole-5-carbaldehyde o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50011780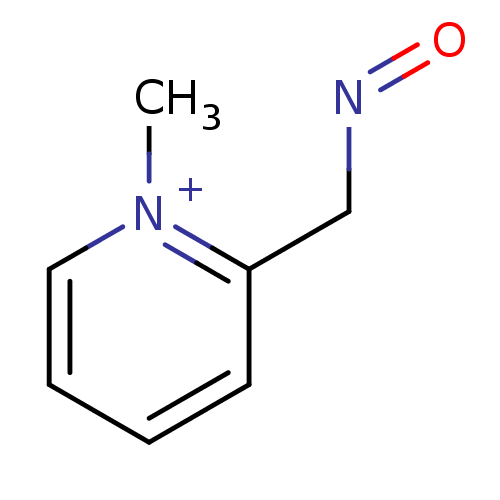 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025246 (2-[2-(4-Carbamoyl-pyridin-1-yloxy)-ethyl]-6-(hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025241 (3-Thiophen-2-yl-[1,2,4]oxadiazole-5-carbaldehyde o...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025253 (CHEMBL69059 | N-(2-Diethylamino-ethyl)-N'-hydroxy-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025244 (3-Phenyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025240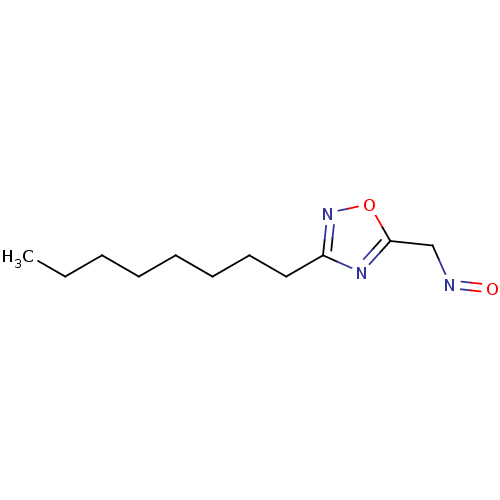 (3-Octyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025243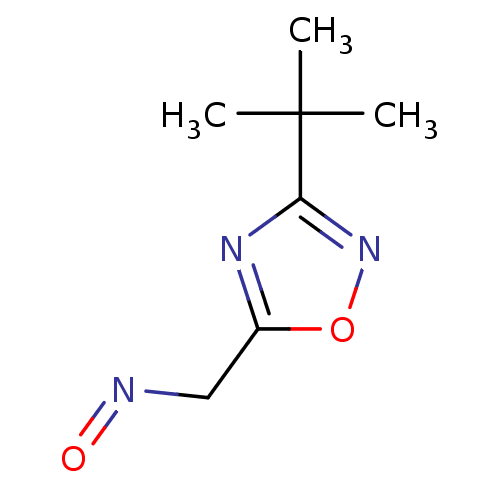 (3-tert-Butyl-[1,2,4]oxadiazole-5-carbaldehyde oxim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025244 (3-Phenyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025245 (3-Pyridin-4-yl-[1,2,4]oxadiazole-5-carbaldehyde ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025249 ((E)-3-Methyl-[1,2,4]oxadiazole-5-carbaldehyde oxim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the concentration required for reversible inhibition of eel acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025250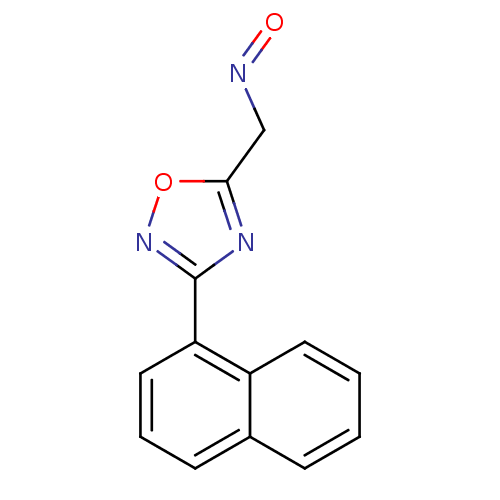 (3-Naphthalen-1-yl-[1,2,4]oxadiazole-5-carbaldehyde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025248 (3-Benzyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025246 (2-[2-(4-Carbamoyl-pyridin-1-yloxy)-ethyl]-6-(hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025248 (3-Benzyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025249 ((E)-3-Methyl-[1,2,4]oxadiazole-5-carbaldehyde oxim...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025240 (3-Octyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025243 (3-tert-Butyl-[1,2,4]oxadiazole-5-carbaldehyde oxim...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025249 ((E)-3-Methyl-[1,2,4]oxadiazole-5-carbaldehyde oxim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025249 ((E)-3-Methyl-[1,2,4]oxadiazole-5-carbaldehyde oxim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the concentration required for reversible inhibition of human acetylcholinesterase . | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025244 (3-Phenyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50405148 (CHEMBL280945) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
TBA Curated by ChEMBL | Assay Description Concentration at 2 uM that inhibits 50%of acetylcholinesterase activity evaluated in vitro at a pH of 8 in the presence of 7.5x10E-4 acetylthiocholin... | J Med Chem 27: 1201-11 (1984) BindingDB Entry DOI: 10.7270/Q2XK8GRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025250 (3-Naphthalen-1-yl-[1,2,4]oxadiazole-5-carbaldehyde...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025244 (3-Phenyl-[1,2,4]oxadiazole-5-carbaldehyde oxime | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||