

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50403292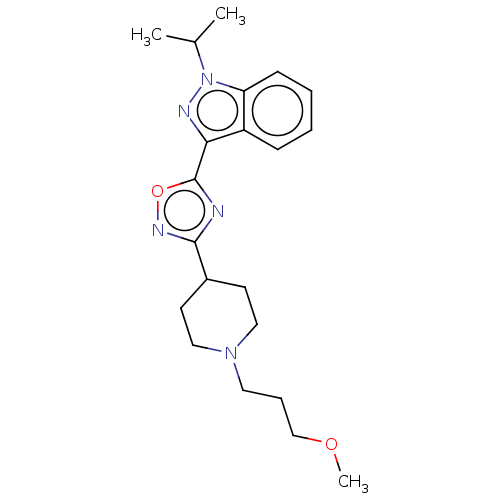 (CHEMBL5285364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391288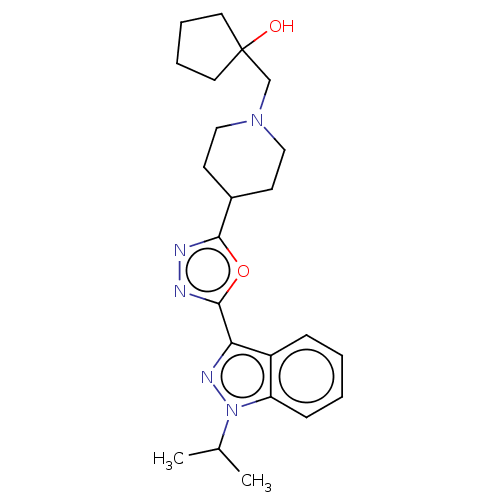 (CHEMBL5288438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50456116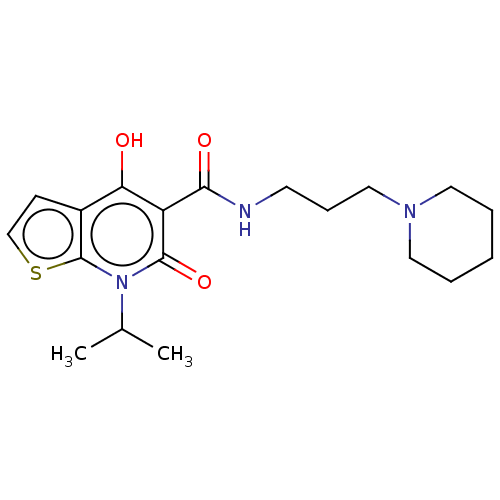 (Prx-03140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391287 (CHEMBL5278176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391258 (CHEMBL5286257) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM325596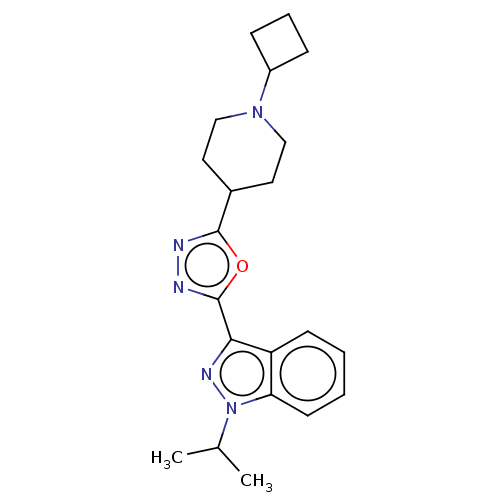 (3-[5-(1-Cyclobutyl-piperidin-4-yl)-[1,3,4]oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391288 (CHEMBL5288438) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391298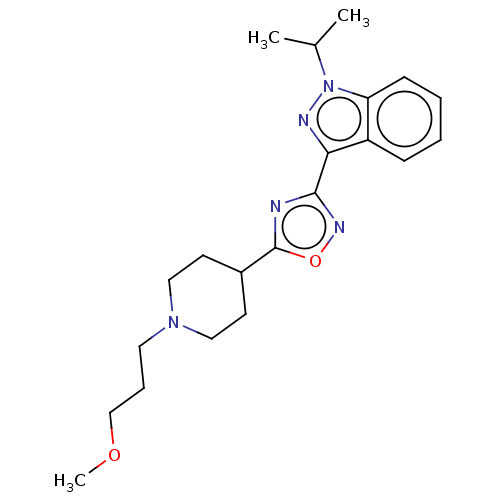 (CHEMBL5275605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM325561 (1-Isopropyl-3-{5-[1-(3-methoxy propyl) piperidin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM325602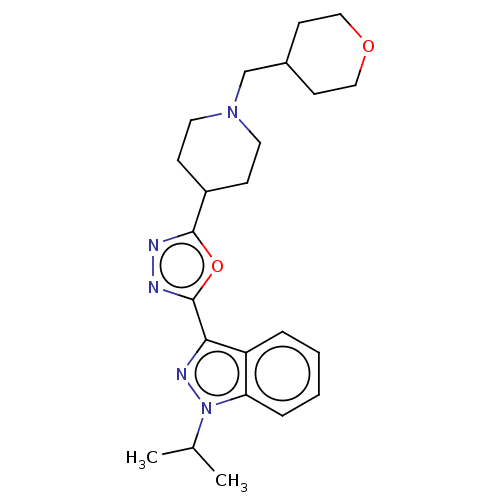 (1-Isopropyl-3-{5-[1-(tetrahydro-pyran-4-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391267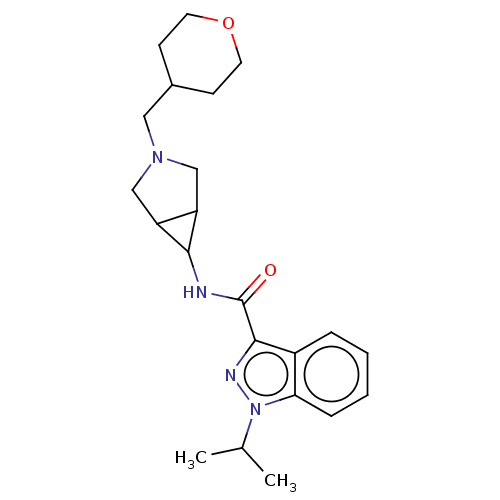 (CHEMBL5289747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM325561 (1-Isopropyl-3-{5-[1-(3-methoxy propyl) piperidin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391246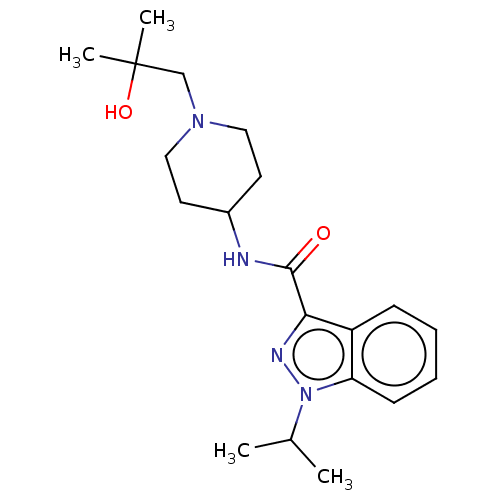 (CHEMBL5275895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rat microsomal HMG-CoA reductase activity by 50% | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391283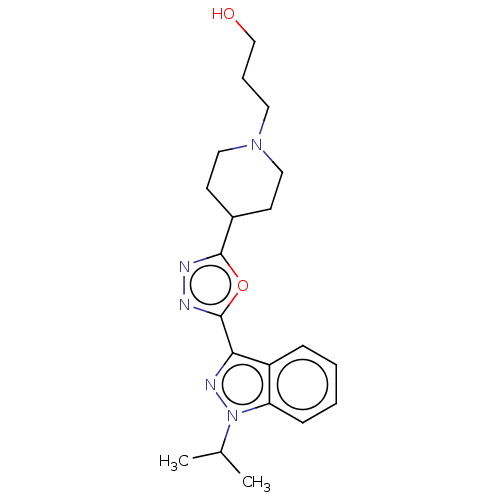 (CHEMBL5268064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Evaluated for inhibitory activity against rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391281 (CHEMBL5286642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391281 (CHEMBL5286642) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391266 (CHEMBL5271080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391278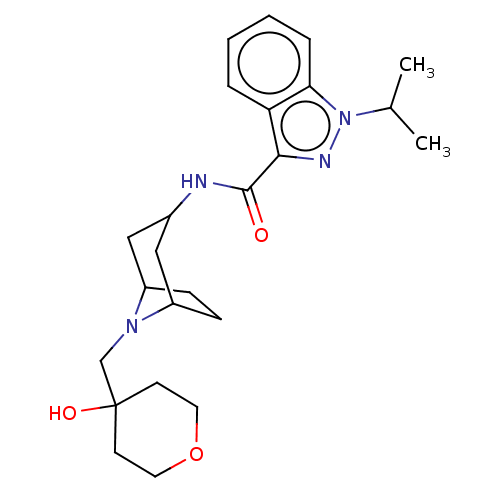 (CHEMBL5274204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM325598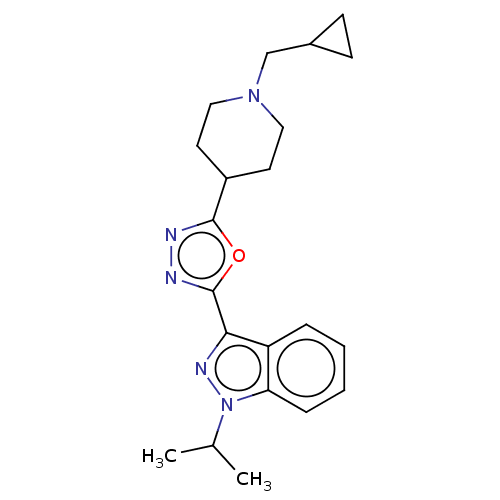 (3-[5-(1-Cyclopropylmethyl-piperidin-4-yl)-[1,3,4]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391265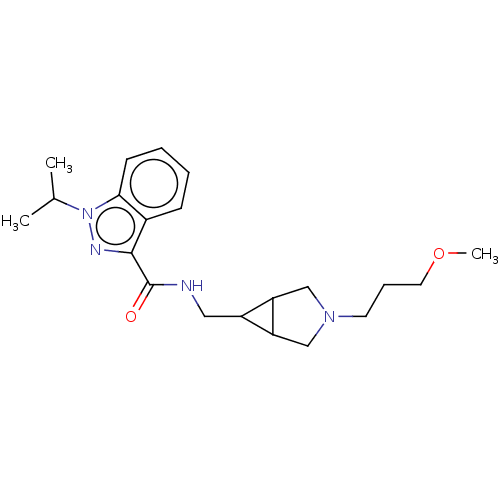 (CHEMBL5276171) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM325596 (3-[5-(1-Cyclobutyl-piperidin-4-yl)-[1,3,4]oxadiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50403292 (CHEMBL5285364) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-2 adrenergic receptor from guinea pig trachea. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391298 (CHEMBL5275605) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for alpha-adrenergic activity against Alpha-1 adrenergic receptor from rat aorta. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM325602 (1-Isopropyl-3-{5-[1-(tetrahydro-pyran-4-ylmethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM325561 (1-Isopropyl-3-{5-[1-(3-methoxy propyl) piperidin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391283 (CHEMBL5268064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM325594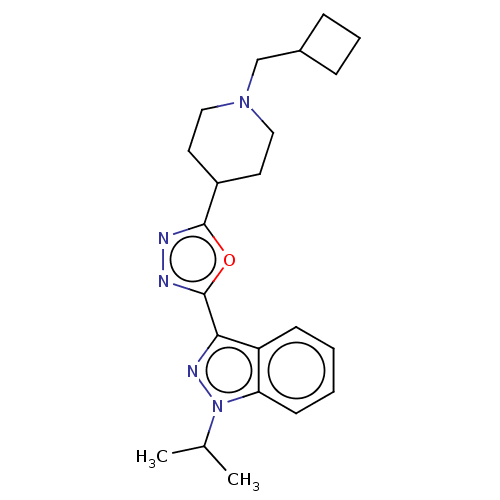 (3-[5-(1-Cyclobutylmethyl-piperidin-4-yl)-[1,3,4]ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM325597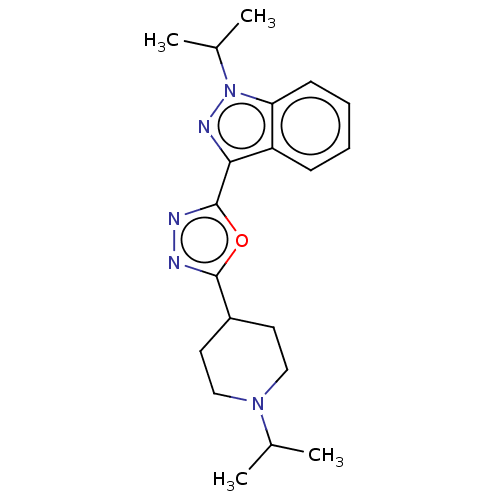 (1-Isopropyl-3-[5-(1-isopropyl-piperidin-4-yl)-[1,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391278 (CHEMBL5274204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391267 (CHEMBL5289747) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against Thromboxane A2 receptor in guinea pig trachea in the presence of 11,9-epoxymethano-PGH2 | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391266 (CHEMBL5271080) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391265 (CHEMBL5276171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391248 (CHEMBL5284140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Evaluated for inhibitory activity against rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50391246 (CHEMBL5275895) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50403292 (CHEMBL5285364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391298 (CHEMBL5275605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391288 (CHEMBL5288438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM325602 (1-Isopropyl-3-{5-[1-(tetrahydro-pyran-4-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM325596 (3-[5-(1-Cyclobutyl-piperidin-4-yl)-[1,3,4]oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM325598 (3-[5-(1-Cyclopropylmethyl-piperidin-4-yl)-[1,3,4]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM325594 (3-[5-(1-Cyclobutylmethyl-piperidin-4-yl)-[1,3,4]ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM325597 (1-Isopropyl-3-[5-(1-isopropyl-piperidin-4-yl)-[1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391248 (CHEMBL5284140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391246 (CHEMBL5275895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50391283 (CHEMBL5268064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50391258 (CHEMBL5286257) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Beta-1 adrenergic receptor activitation measured by isoprenaline-induced positive inotropic effect in guinea pig left atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50391255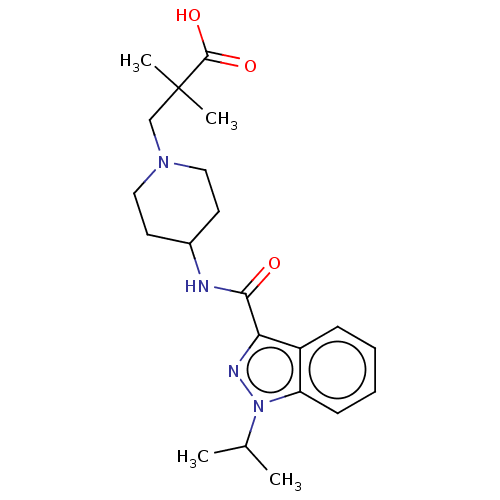 (CHEMBL5283568) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Beta-1 adrenergic receptor activitation measured by isoprenaline-induced positive inotropic effect in guinea pig left atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50391256 (CHEMBL5271303) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Beta-1 adrenergic receptor activitation measured by isoprenaline-induced positive inotropic effect in guinea pig left atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50391259 (CHEMBL5290222) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Beta-1 adrenergic receptor activitation measured by isoprenaline-induced positive inotropic effect in guinea pig left atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50391260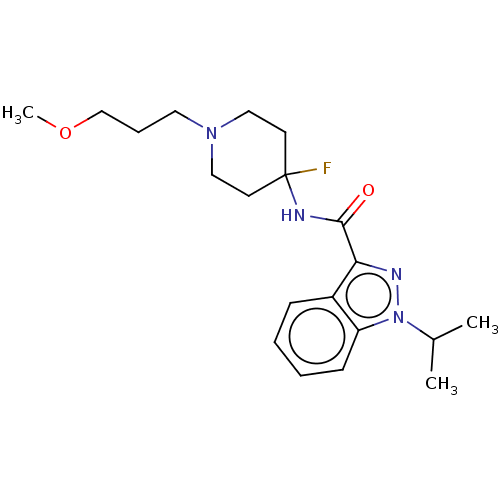 (CHEMBL5288891) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Beta-1 adrenergic receptor activitation measured by isoprenaline-induced positive inotropic effect in guinea pig left atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |