

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291573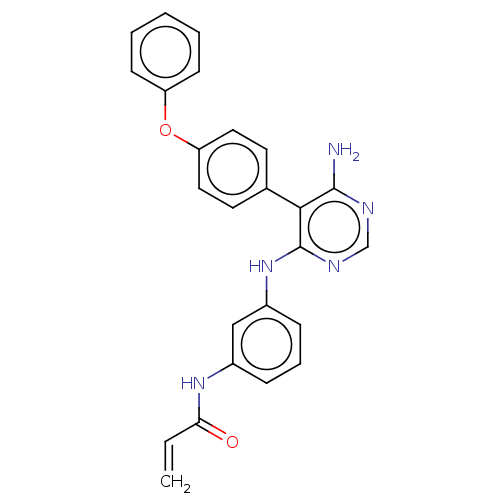 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291413 (1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291455 (N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291452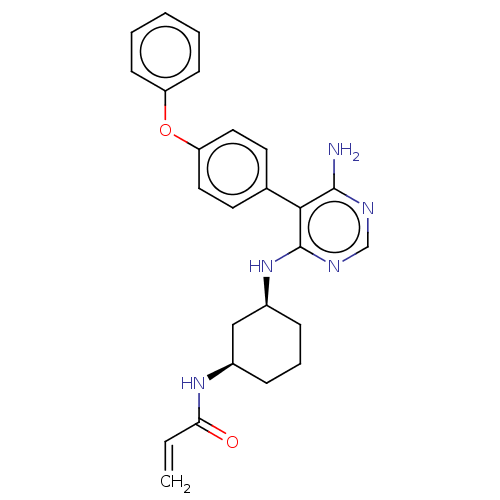 (N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50463725 (CHEMBL4242598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291522 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291635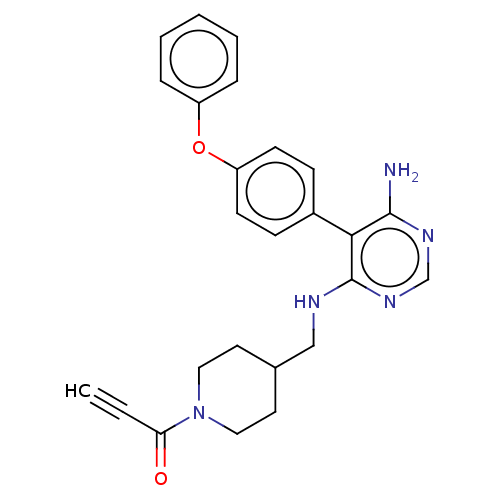 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50519156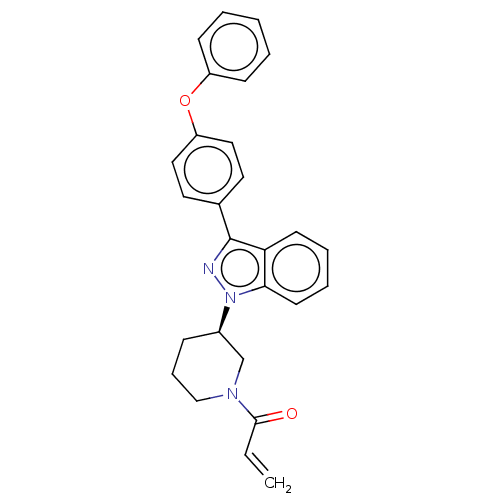 (CHEMBL4466205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291634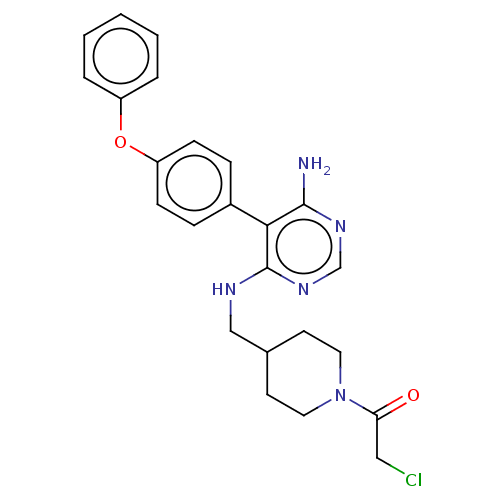 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50463725 (CHEMBL4242598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466204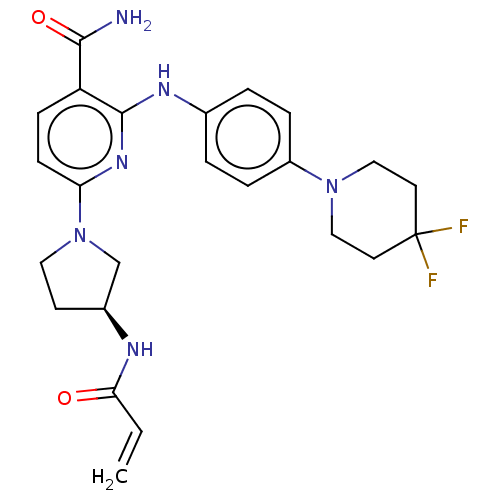 (CHEMBL4288997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291389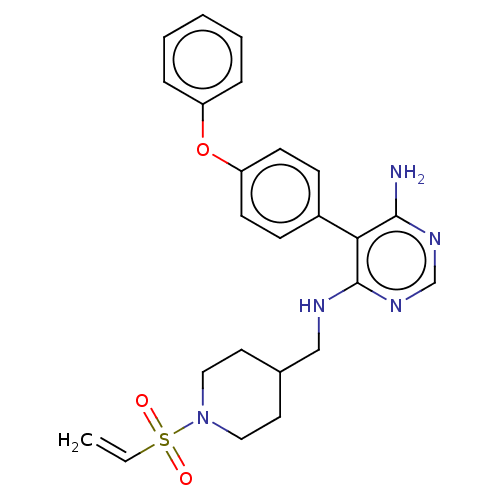 (5-(4-phenoxyphenyl)-N4-((1-(vinylsulfonyl)piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM291512 (N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466202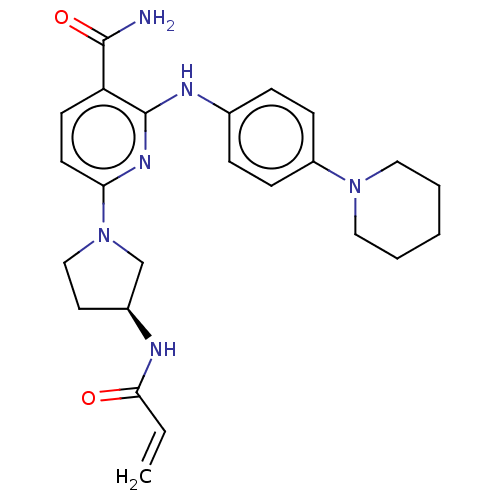 (CHEMBL4278321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466206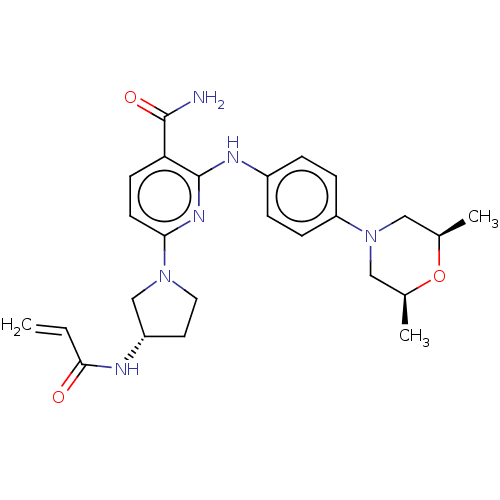 (CHEMBL4281335) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50463727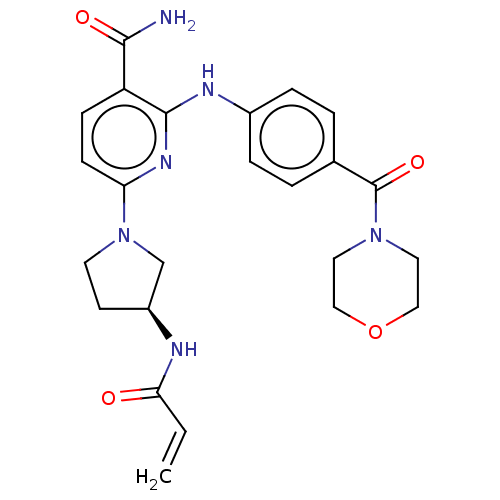 (CHEMBL4245727) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50463727 (CHEMBL4245727) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466208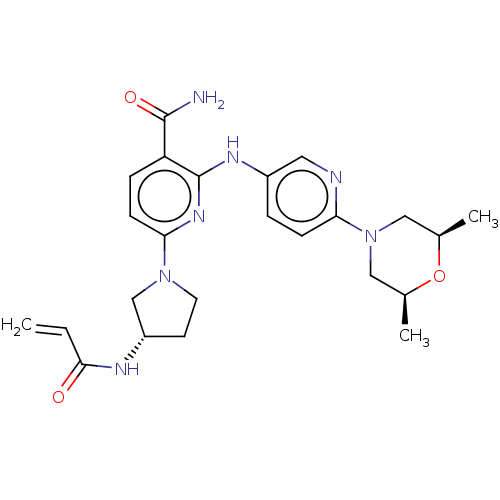 (CHEMBL4282893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291413 (1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466203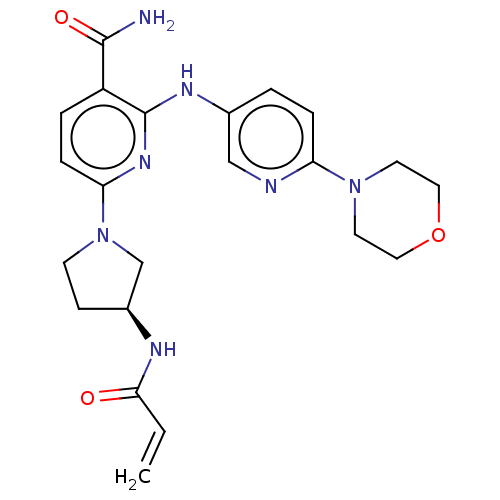 (CHEMBL4286628) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466207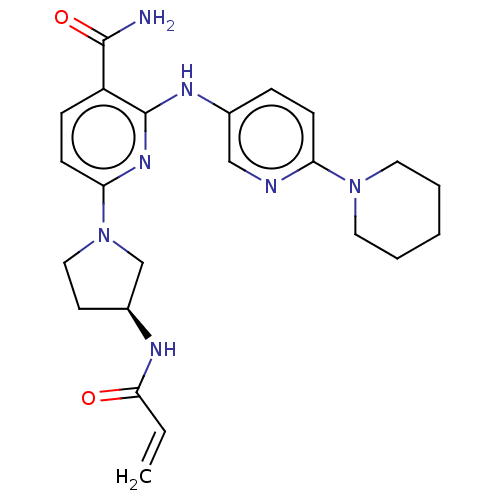 (CHEMBL4278717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466205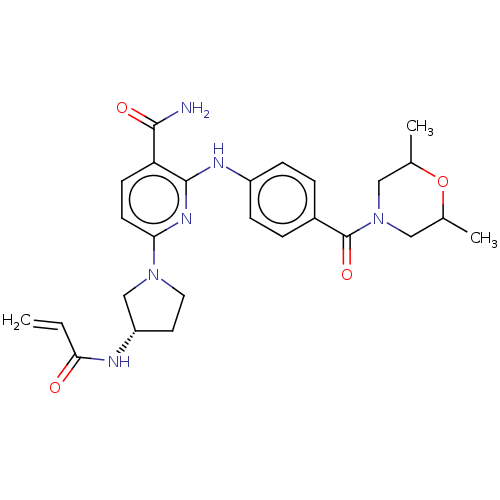 (CHEMBL4285083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291513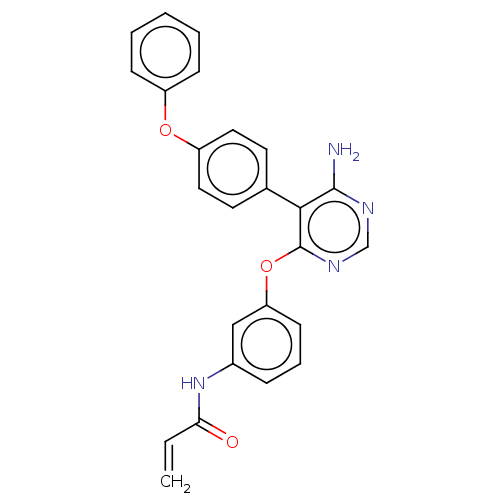 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291573 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM291573 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50463723 (CHEMBL4250200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50463729 (CHEMBL4246399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC assessed as reduction in anti-IgM-induced CD69 expression incubated for 1 hr by flow cytometric analysis | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291515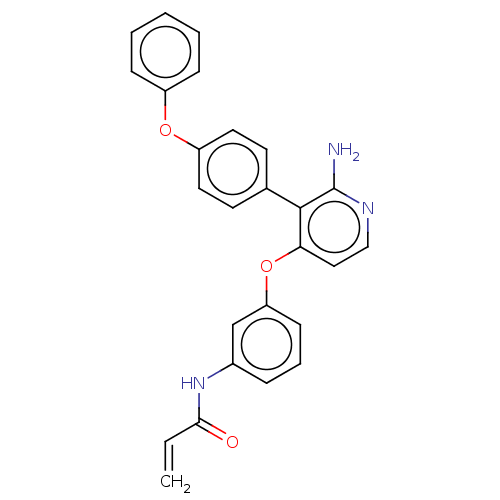 (N-(3-((2-amino-3-(4-phenoxyphenyl)pyridin-4-yl)oxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291425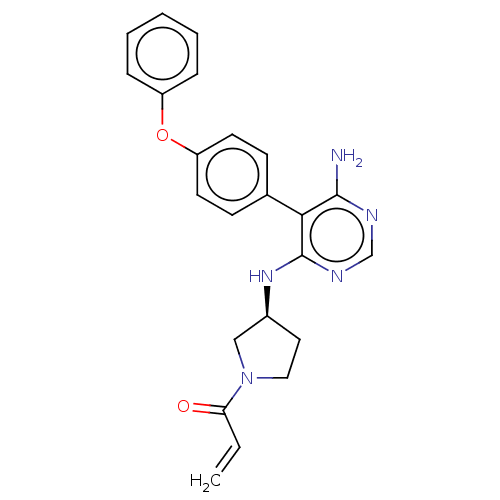 ((S)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291625 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291549 ((S)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50466209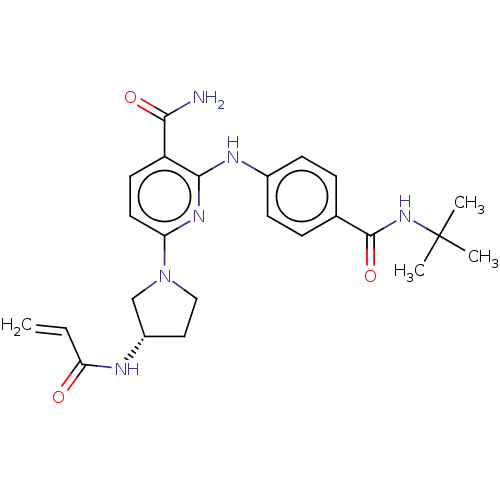 (CHEMBL4290066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay | Bioorg Med Chem Lett 28: 3307-3311 (2018) Article DOI: 10.1016/j.bmcl.2018.09.018 BindingDB Entry DOI: 10.7270/Q2J96914 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291512 (N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291522 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291610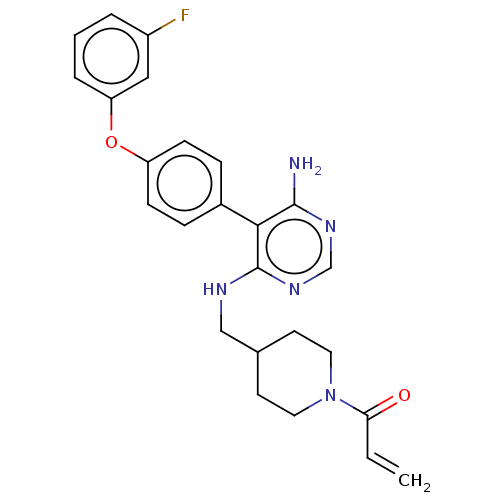 (1-(4-(((6-amino-5-(4-(3-fluorophenoxy)phenyl)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50463725 (CHEMBL4242598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc. Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC assessed as reduction in anti-IgM-induced CD69 expression incubated for 1 hr by flow cytometric analysis | Bioorg Med Chem Lett 28: 2939-2944 (2018) Article DOI: 10.1016/j.bmcl.2018.07.008 BindingDB Entry DOI: 10.7270/Q2QR50TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291513 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291455 (N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378834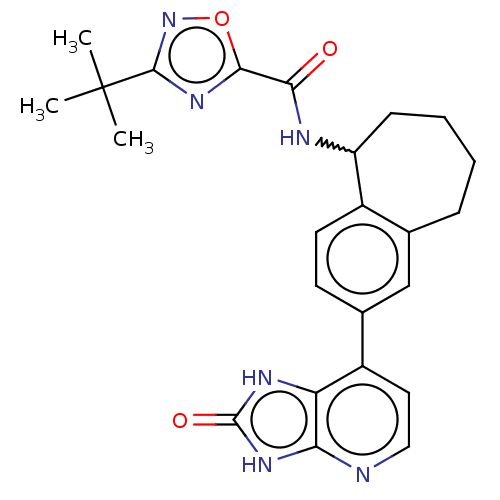 (US10266528, Compound 12 | US10266528, Compound 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378835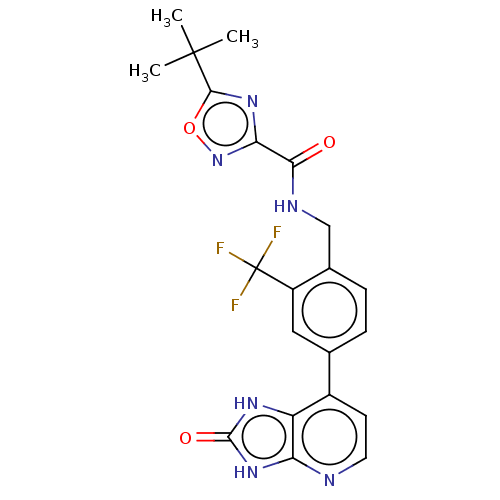 (US10266528, Compound 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378836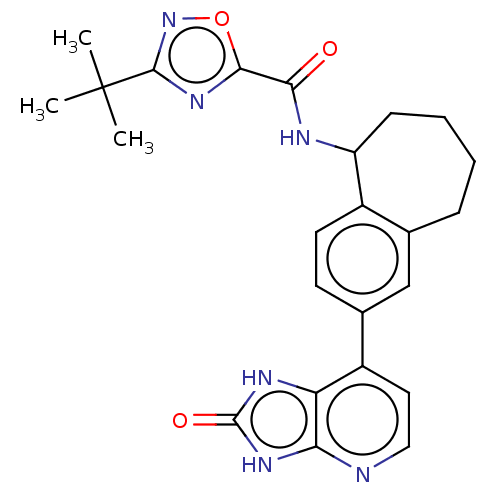 (US10266528, Compound 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378837 (US10266528, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378838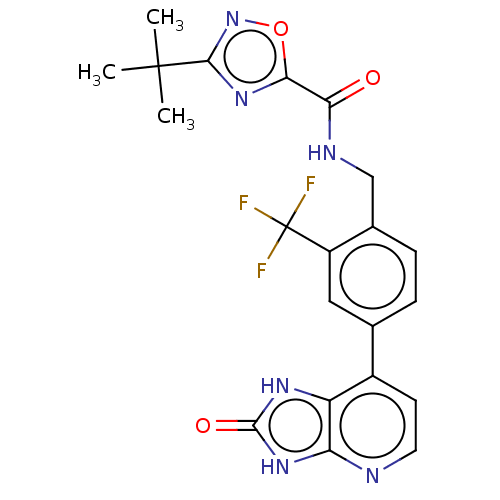 (US10266528, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378839 (US10266528, Compound 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378840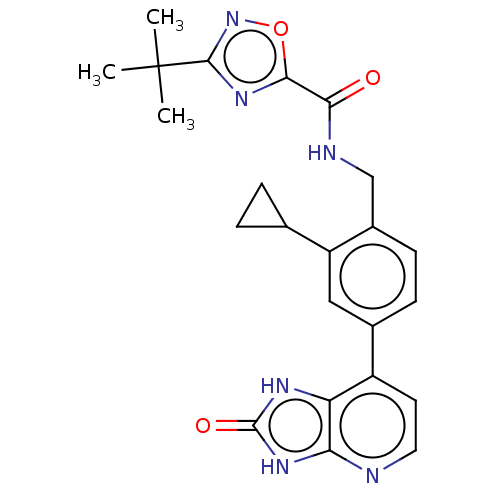 (US10266528, Compound 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378841 (US10266528, Compound 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Briefly, 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC... | Bioorg Med Chem Lett 17: 5630-3 (2007) BindingDB Entry DOI: 10.7270/Q28S4S6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 625 total ) | Next | Last >> |