 Found 109 hits with Last Name = 'guido' and Initial = 'rvc'
Found 109 hits with Last Name = 'guido' and Initial = 'rvc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50567995
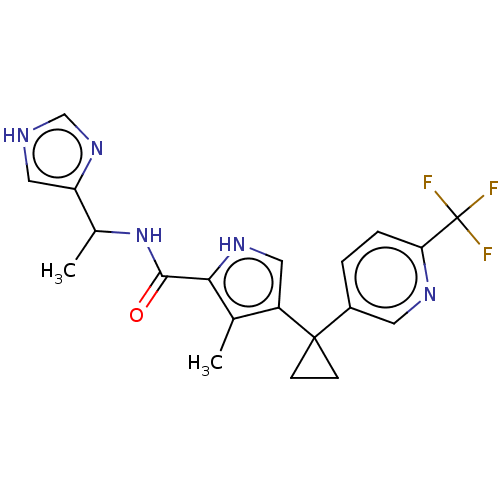
(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50567966
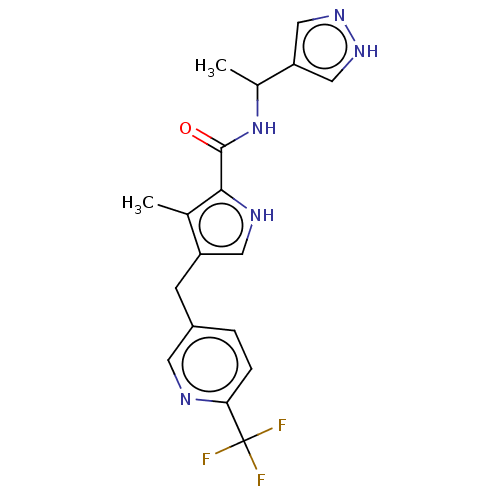
(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50567998
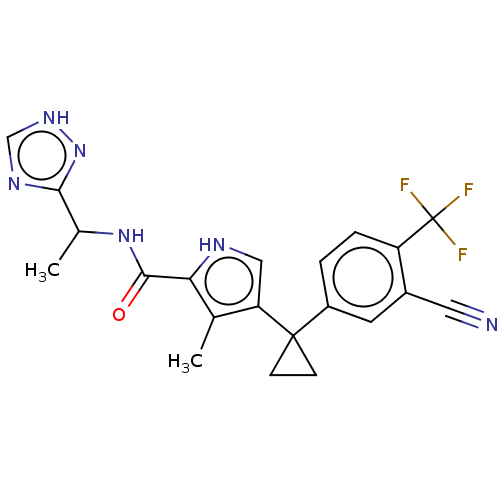
(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NK1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50567966

(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50567966

(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50567966

(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50567966

(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50567966

(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50567966

(CHEMBL4850116)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cn[nH]c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG by QPatch assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567957

(CHEMBL4855439)Show SMILES COc1ccc(Cc2c[nH]c(C(=O)NC3CC3)c2C)c2cnccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50365224
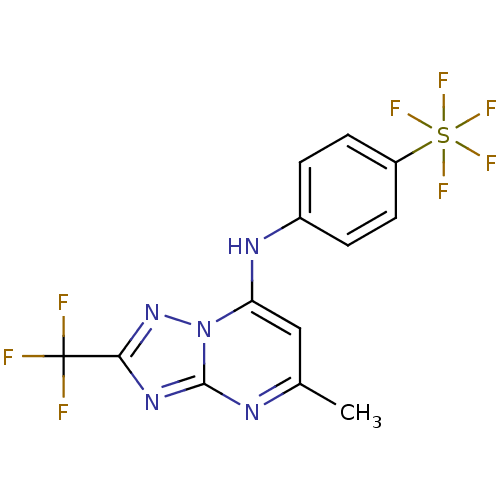
(CHEMBL1956291 | US9238653, Table 5, Compound 29)Show SMILES Cc1cc(Nc2ccc(cc2)S(F)(F)(F)(F)F)n2nc(nc2n1)C(F)(F)F Show InChI InChI=1S/C13H9F8N5S/c1-7-6-10(26-12(22-7)24-11(25-26)13(14,15)16)23-8-2-4-9(5-3-8)27(17,18,19,20)21/h2-6,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50538344
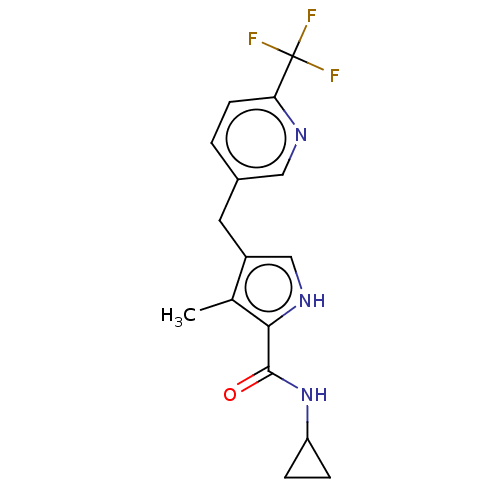
(CHEMBL4633246)Show SMILES Cc1c(Cc2ccc(nc2)C(F)(F)F)c[nH]c1C(=O)NC1CC1 Show InChI InChI=1S/C16H16F3N3O/c1-9-11(8-21-14(9)15(23)22-12-3-4-12)6-10-2-5-13(20-7-10)16(17,18)19/h2,5,7-8,12,21H,3-4,6H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50538359

(CHEMBL4635646)Show SMILES Cc1c(Cc2ccc(cc2F)C(F)(F)F)c[nH]c1C(=O)NC1CC1 Show InChI InChI=1S/C17H16F4N2O/c1-9-11(8-22-15(9)16(24)23-13-4-5-13)6-10-2-3-12(7-14(10)18)17(19,20)21/h2-3,7-8,13,22H,4-6H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567951

(CHEMBL4855152)Show SMILES Cc1c(Cc2ccc(nc2F)C(F)(F)F)c[nH]c1C(=O)NC1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567952

(CHEMBL4878579)Show SMILES Cc1c(Cc2ccc(nc2F)C(F)(F)F)c[nH]c1C(=O)NCC(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567953

(CHEMBL4878940)Show SMILES Cc1c(Cc2ccc(nc2F)C(F)(F)F)c[nH]c1C(=O)N1CC(F)(F)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567954

(CHEMBL4856991) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567955

(CHEMBL4855268)Show SMILES Cc1c(Cc2cccc3ccncc23)c[nH]c1C(=O)N1CC(F)(F)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567956

(CHEMBL4861785)Show SMILES Cc1c(Cc2cccc3ccncc23)c[nH]c1C(=O)NCC(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567958

(CHEMBL4847032) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567959

(CHEMBL4847652)Show SMILES Cc1c(Cc2ccc(F)c3ccncc23)c[nH]c1C(=O)N1CC(F)(F)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567960

(CHEMBL4877462)Show SMILES CCOC(=O)c1[nH]cc(Cc2ccc(c3ncoc23)C(F)(F)F)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567961

(CHEMBL4872774) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567962

(CHEMBL4846095) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567963
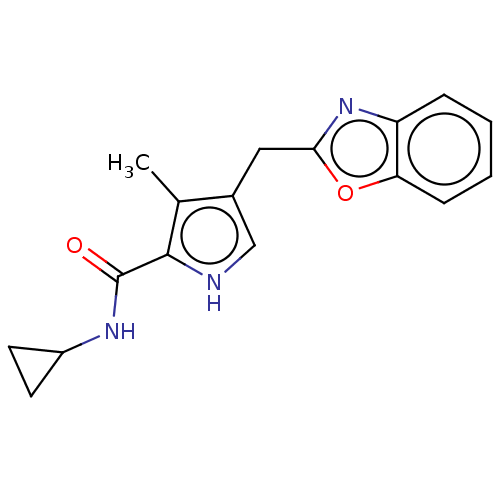
(CHEMBL4874350) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50567964

(CHEMBL4858336 | US11903936, Compound 29)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cc(C)on1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00173
BindingDB Entry DOI: 10.7270/Q2NZ8CD4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data