

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100403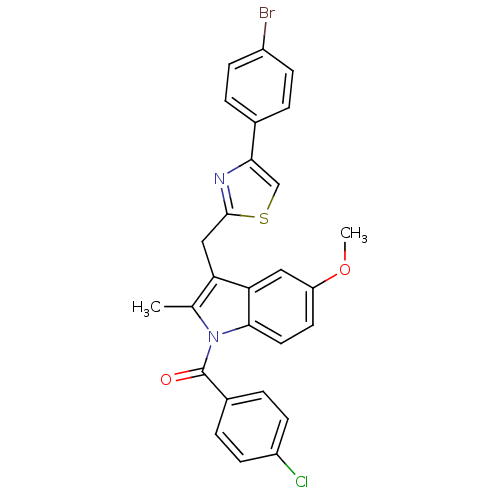 (CHEMBL423296 | {3-[4-(4-Bromo-phenyl)-thiazol-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100395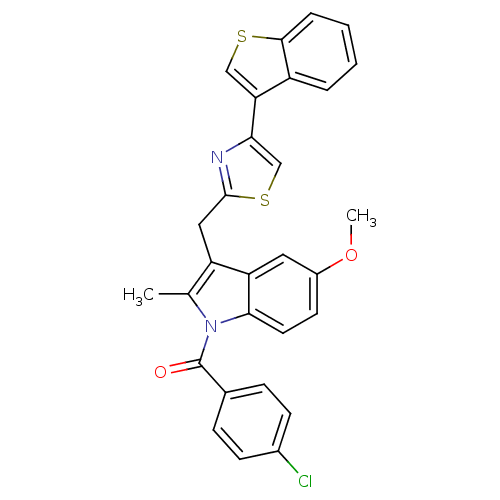 (CHEMBL28155 | [3-(4-Benzo[b]thiophen-3-yl-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100404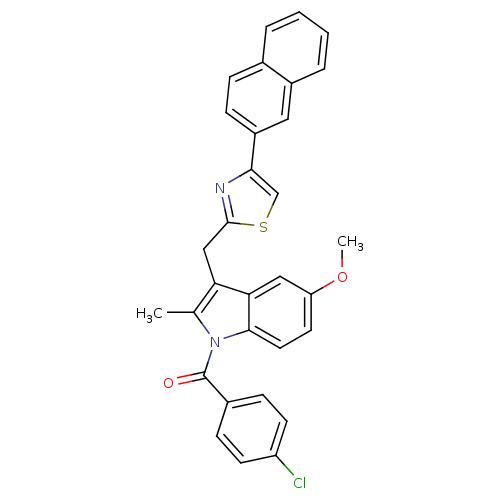 ((4-Chloro-phenyl)-[5-methoxy-2-methyl-3-(4-naphtha...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100401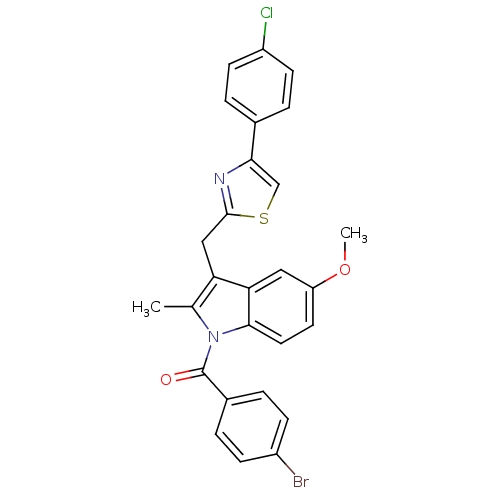 ((4-Bromo-phenyl)-{3-[4-(4-chloro-phenyl)-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100400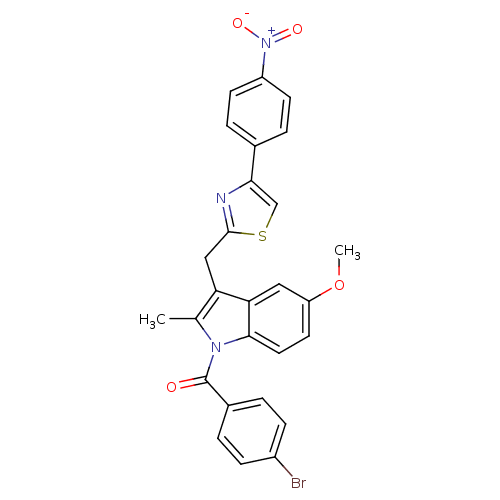 ((4-Bromo-phenyl)-{5-methoxy-2-methyl-3-[4-(4-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100396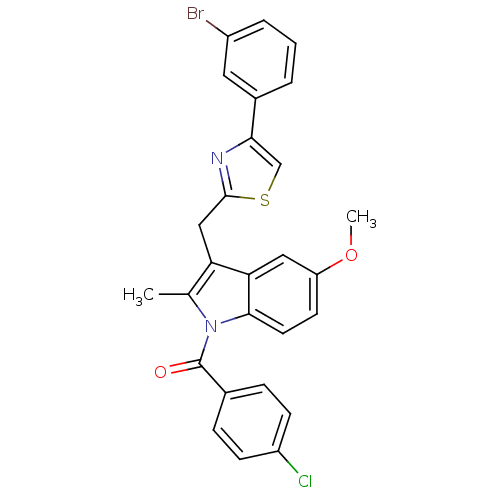 (CHEMBL24407 | {3-[4-(3-Bromo-phenyl)-thiazol-2-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100399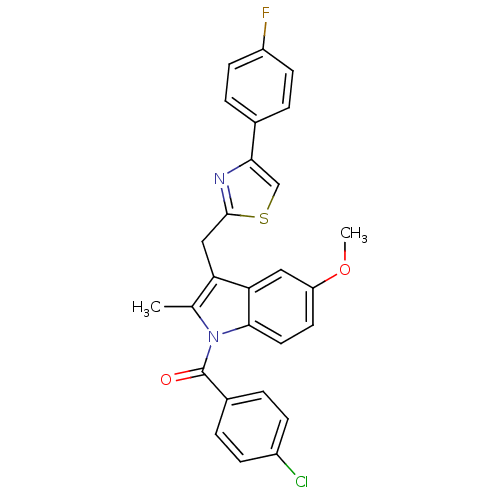 ((4-Chloro-phenyl)-{3-[4-(4-fluoro-phenyl)-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100402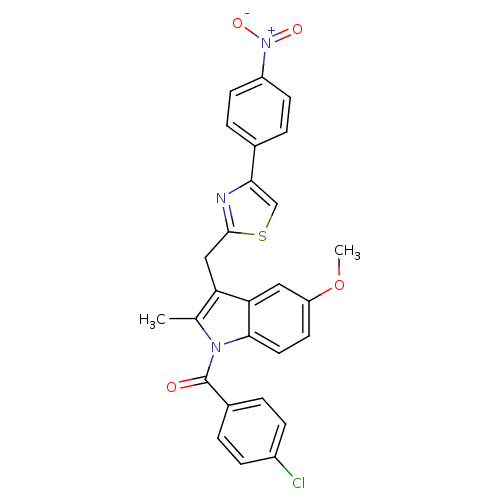 ((4-Chloro-phenyl)-{5-methoxy-2-methyl-3-[4-(4-nitr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100397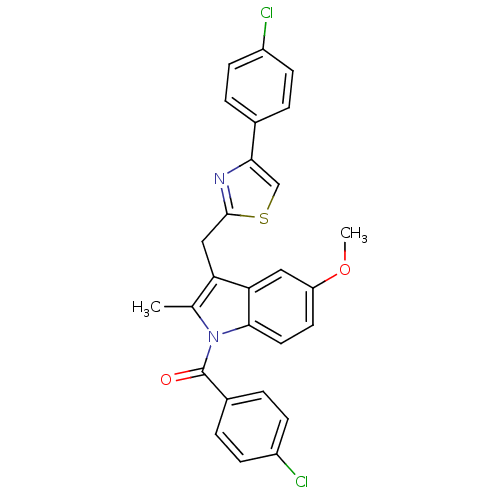 ((4-Chloro-phenyl)-{3-[4-(4-chloro-phenyl)-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100405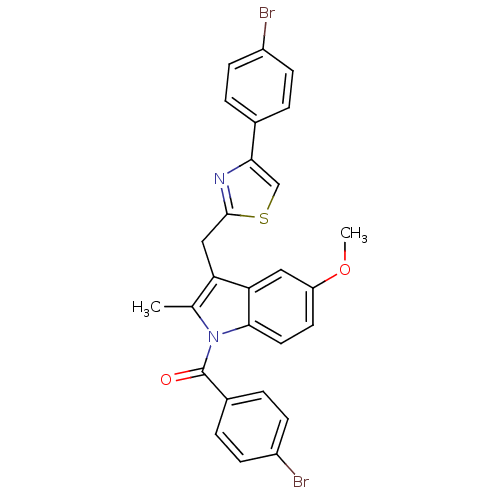 ((4-Bromo-phenyl)-{3-[4-(4-bromo-phenyl)-thiazol-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100398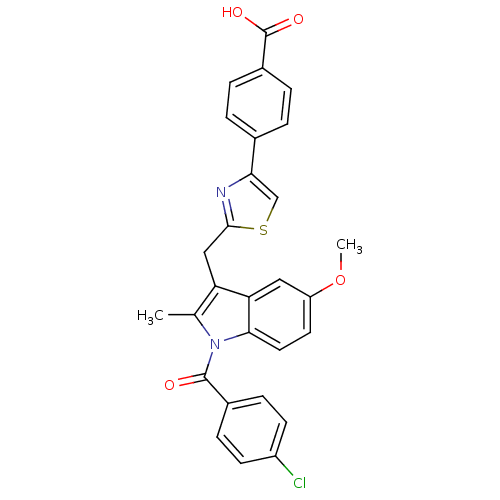 (4-{2-[1-(4-Chloro-benzoyl)-5-methoxy-2-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50100394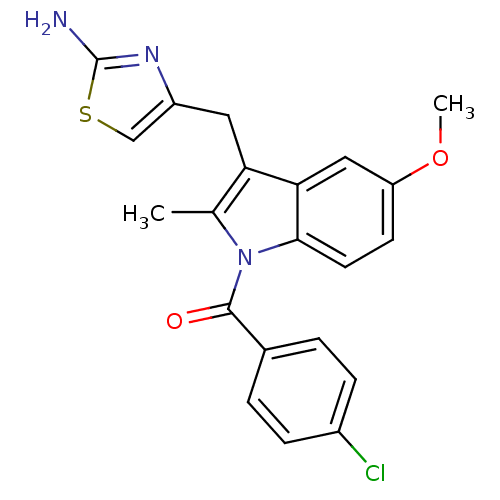 (CHEMBL24687 | [3-(2-Amino-thiazol-4-ylmethyl)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Prostaglandin G/H synthase 2 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111568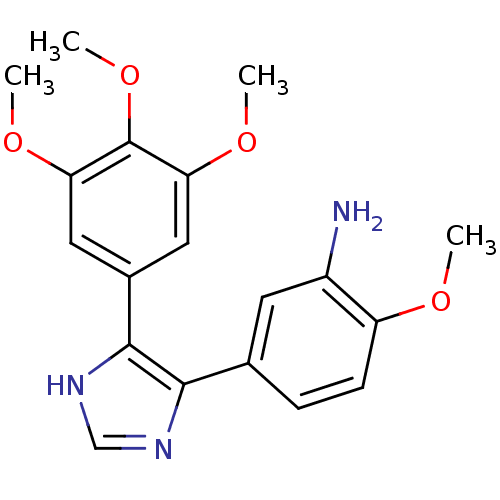 (2-Methoxy-5-[5-(3,4,5-trimethoxy-phenyl)-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111579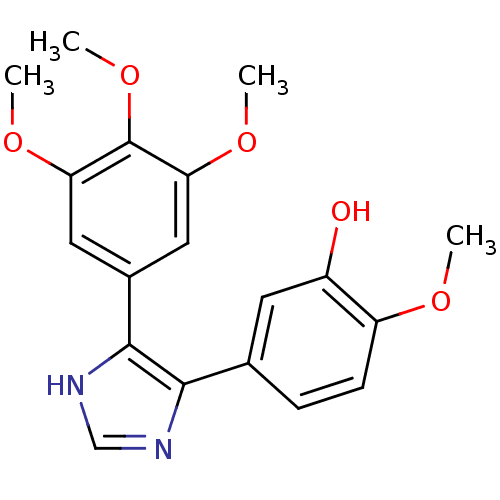 (2-Methoxy-5-[5-(3,4,5-trimethoxy-phenyl)-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111574 (1-Methyl-5-[4-(3,4,5-trimethoxy-phenyl)-oxazol-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111554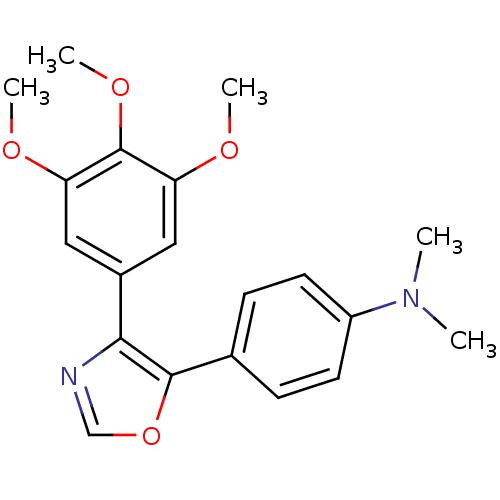 (CHEMBL296066 | Dimethyl-{4-[4-(3,4,5-trimethoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111558 (2-Methoxy-5-[4-(3,4,5-trimethoxy-phenyl)-oxazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111567 (2-Methoxy-5-[4-(3,4,5-trimethoxy-phenyl)-oxazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111566 (1-Methyl-6-[5-(3,4,5-trimethoxy-phenyl)-3H-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111555 (1-Methyl-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111569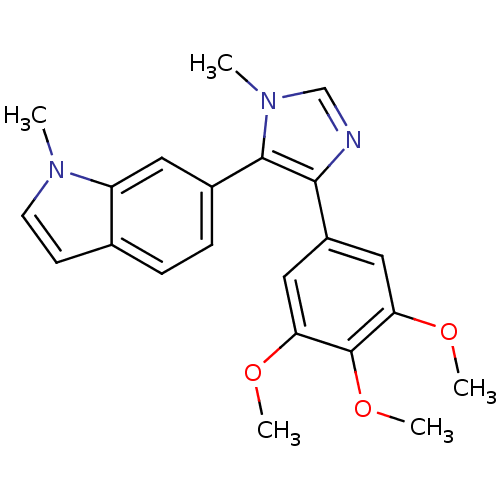 (1-Methyl-6-[3-methyl-5-(3,4,5-trimethoxy-phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111580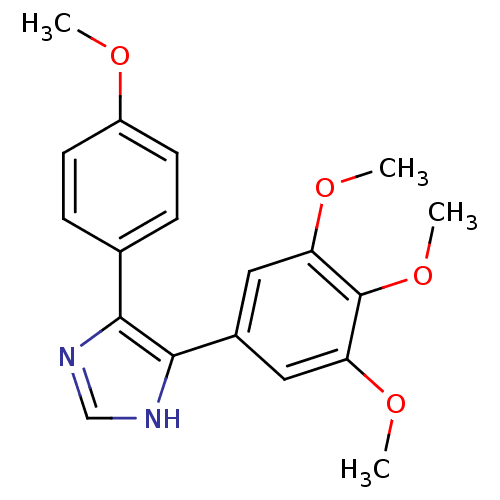 (5-(4-Methoxy-phenyl)-4-(3,4,5-trimethoxy-phenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111570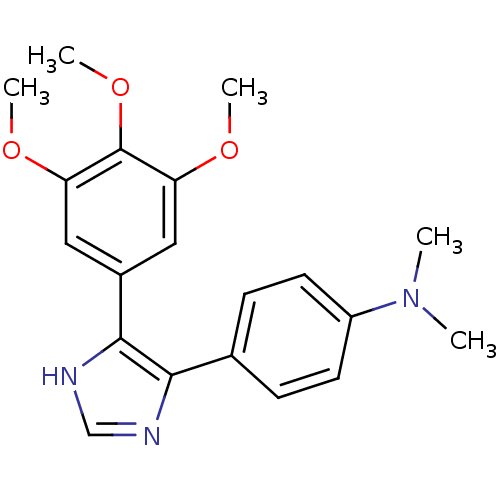 (CHEMBL296406 | Dimethyl-{4-[5-(3,4,5-trimethoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111578 (2-Methoxy-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111562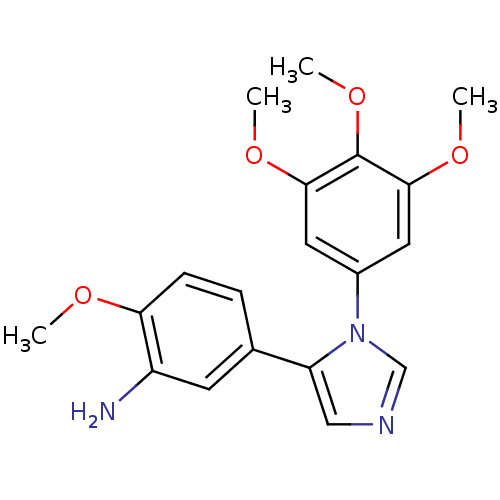 (2-Methoxy-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against human recombinant Prostaglandin G/H synthase 1 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111556 (CHEMBL297236 | Dimethyl-{4-[3-(3,4,5-trimethoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111559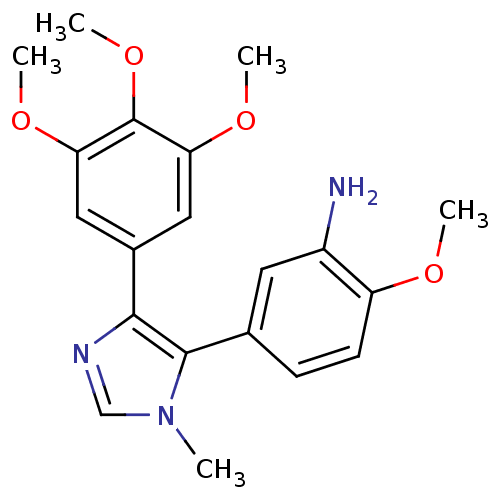 (2-Methoxy-5-[3-methyl-5-(3,4,5-trimethoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111561 (CHEMBL47112 | Dimethyl-{4-[1-(3,4,5-trimethoxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111582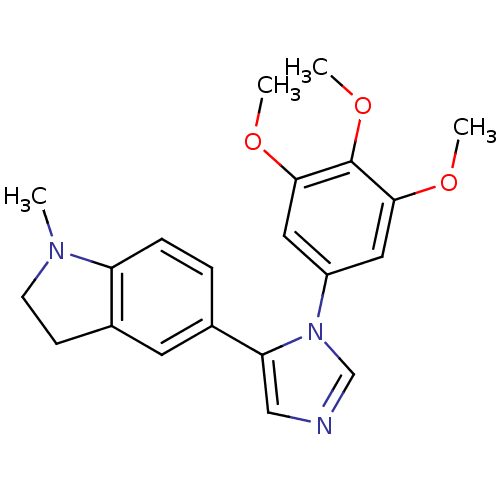 (1-Methyl-5-[3-(3,4,5-trimethoxy-phenyl)-3H-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111565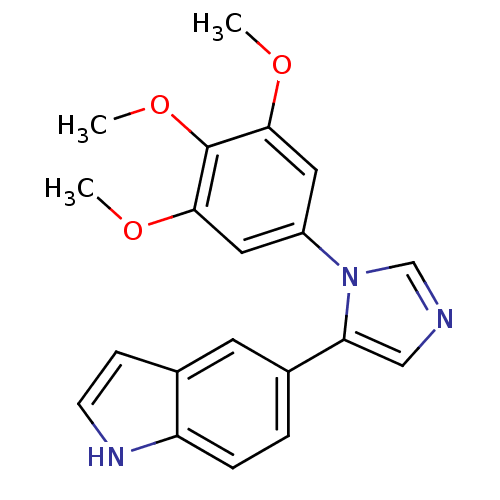 (5-[3-(3,4,5-Trimethoxy-phenyl)-3H-imidazol-4-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50111576 (CHEMBL297018 | Dimethyl-{4-[5-(3,4,5-trimethoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration needed to inhibit tubulin polymerization by 50% | J Med Chem 45: 1697-711 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50100394 (CHEMBL24687 | [3-(2-Amino-thiazol-4-ylmethyl)-5-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.55E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against human recombinant Prostaglandin G/H synthase 1 cloned and expressed in baculovirus (Sf9) | Bioorg Med Chem Lett 11: 1325-8 (2001) BindingDB Entry DOI: 10.7270/Q2PG1R1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||