 Found 47 hits with Last Name = 'bickerton' and Initial = 's'
Found 47 hits with Last Name = 'bickerton' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580186
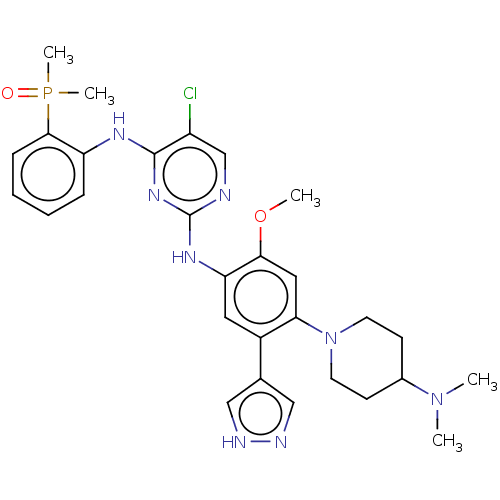
(CHEMBL5094480)Show SMILES COc1cc(N2CCC(CC2)N(C)C)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580187
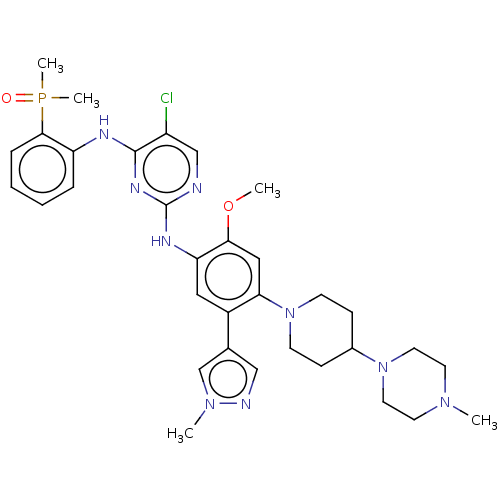
(CHEMBL5087815)Show SMILES COc1cc(N2CCC(CC2)N2CCN(C)CC2)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cnn(C)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580185
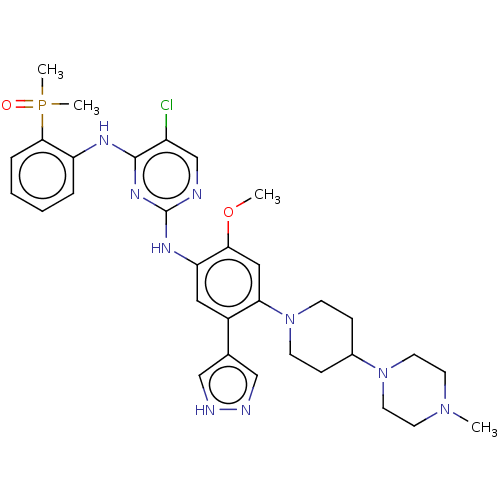
(CHEMBL5077378)Show SMILES COc1cc(N2CCC(CC2)N2CCN(C)CC2)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580188

(CHEMBL5086066)Show SMILES COc1cc(N2CCC(CC2)N(C)C)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cnn(C)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580192
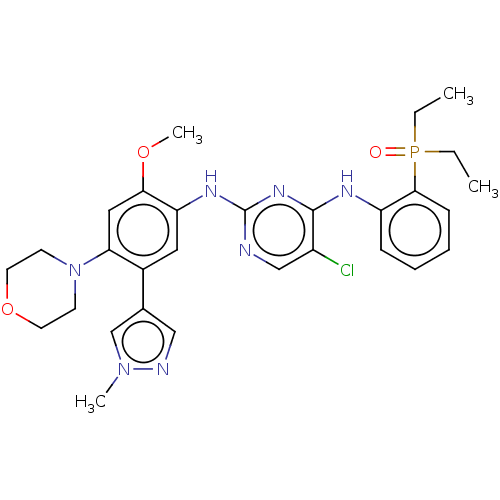
(CHEMBL5078413)Show SMILES CCP(=O)(CC)c1ccccc1Nc1nc(Nc2cc(-c3cnn(C)c3)c(cc2OC)N2CCOCC2)ncc1Cl | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580189
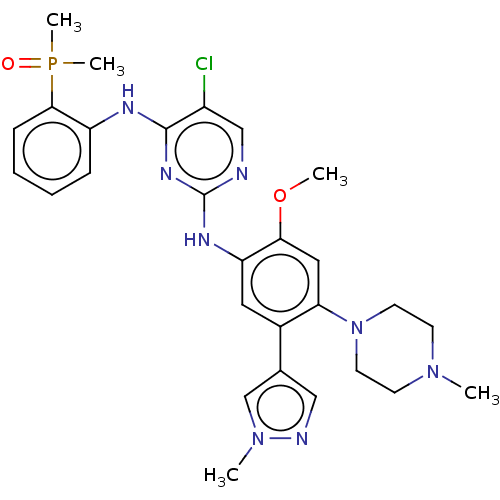
(CHEMBL5087610)Show SMILES COc1cc(N2CCN(C)CC2)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cnn(C)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580190
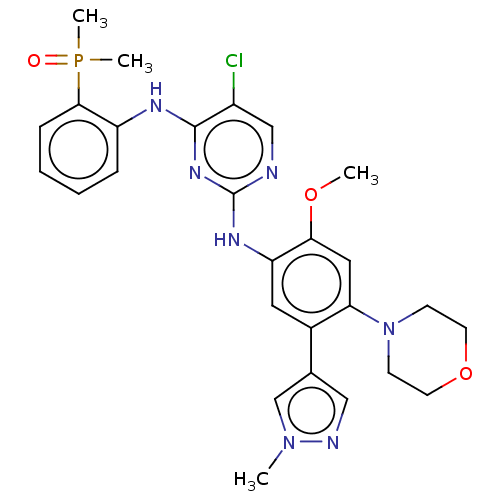
(CHEMBL5087264)Show SMILES COc1cc(N2CCOCC2)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cnn(C)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605543

(CHEMBL5207711)Show SMILES [H][C@]12CN(CCN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527057

(CHEMBL4461434)Show SMILES [H][C@]12CN([C@H](C)CN1c1c(cnc3c(F)c(c(Cl)cc13)-c1c(O)cccc1F)N(C)C2=O)C(=O)C=C |r,wU:1.0,4.4,(50.79,-26.84,;49.46,-27.62,;49.45,-26.08,;48.12,-25.32,;46.79,-26.1,;45.45,-25.34,;46.8,-27.64,;48.13,-28.4,;48.14,-29.94,;49.48,-30.7,;49.49,-32.25,;48.15,-33.03,;46.82,-32.26,;45.48,-33.04,;45.49,-34.58,;44.15,-32.27,;44.15,-30.72,;42.82,-29.95,;45.48,-29.95,;46.81,-30.71,;42.82,-33.04,;41.49,-32.26,;41.49,-30.72,;40.15,-33.03,;40.15,-34.57,;41.49,-35.34,;42.82,-34.57,;44.15,-35.34,;50.81,-29.92,;52.14,-30.69,;50.8,-28.38,;52.13,-27.61,;48.11,-23.78,;46.77,-23.02,;49.44,-23,;49.43,-21.46,)| Show InChI InChI=1S/C25H21ClF2N4O3/c1-4-19(34)31-11-17-25(35)30(3)16-9-29-23-13(24(16)32(17)10-12(31)2)8-14(26)20(22(23)28)21-15(27)6-5-7-18(21)33/h4-9,12,17,33H,1,10-11H2,2-3H3/t12-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580191
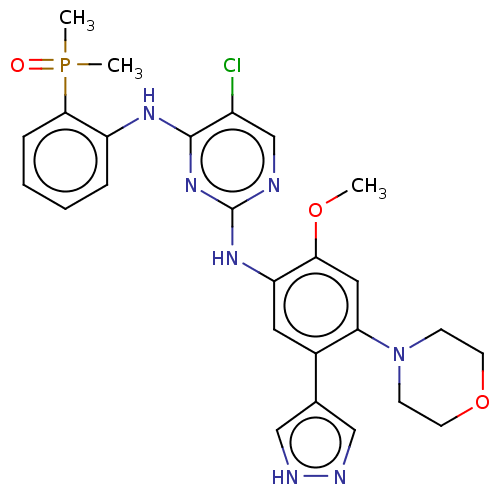
(CHEMBL5081299)Show SMILES COc1cc(N2CCOCC2)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605540
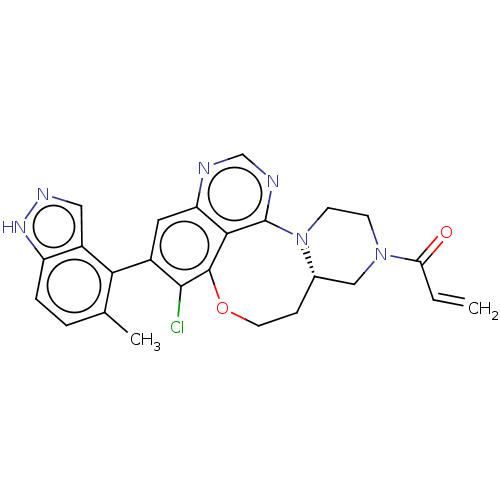
(CHEMBL5171553)Show SMILES [H][C@]12CN(CCN1c1ncnc3cc(c(Cl)c(OCC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wD:1.0,(.27,4.4,;1.04,3.07,;1.88,4.35,;3.42,4.26,;4.11,2.89,;3.27,1.6,;1.73,1.69,;1.3,.21,;2.65,-.54,;2.67,-2.08,;1.35,-2.87,;0,-2.12,;-1.32,-2.91,;-2.66,-2.17,;-2.69,-.63,;-4.03,.12,;-1.37,.17,;-1.94,1.5,;-1.83,3.02,;-.41,3.6,;-.02,-.58,;-3.98,-2.96,;-5.33,-2.21,;-5.36,-.67,;-6.65,-3,;-6.62,-4.54,;-5.28,-5.29,;-4.93,-6.79,;-3.4,-6.93,;-2.8,-5.51,;-3.96,-4.5,;4.27,5.55,;3.58,6.93,;5.8,5.46,;6.65,6.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527051
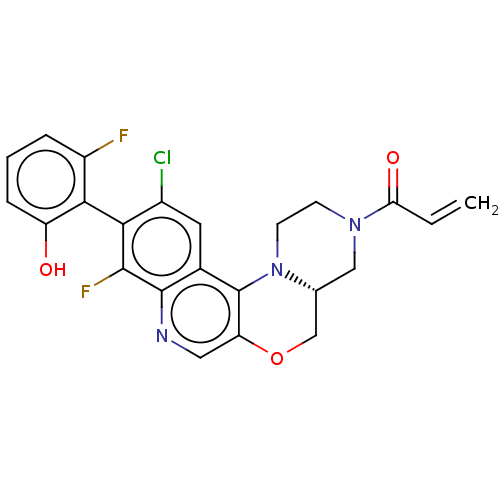
(CHEMBL4591572)Show SMILES [H][C@@]12COc3cnc4c(F)c(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(13.3,-47.65,;11.97,-48.43,;13.31,-49.19,;13.32,-50.73,;11.99,-51.51,;12,-53.06,;10.66,-53.83,;9.33,-53.06,;7.99,-53.84,;8,-55.38,;6.66,-53.07,;6.66,-51.53,;5.33,-50.76,;7.99,-50.76,;9.33,-51.52,;10.65,-50.75,;10.64,-49.21,;9.31,-48.44,;9.3,-46.9,;10.63,-46.13,;11.97,-46.89,;10.62,-44.59,;9.28,-43.82,;11.95,-43.81,;11.94,-42.27,;5.33,-53.84,;4,-53.07,;4,-51.53,;2.66,-53.83,;2.66,-55.38,;4,-56.15,;5.33,-55.37,;6.67,-56.14,)| Show InChI InChI=1S/C23H18ClF2N3O3/c1-2-18(31)28-6-7-29-12(10-28)11-32-17-9-27-22-13(23(17)29)8-14(24)19(21(22)26)20-15(25)4-3-5-16(20)30/h2-5,8-9,12,30H,1,6-7,10-11H2/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527059
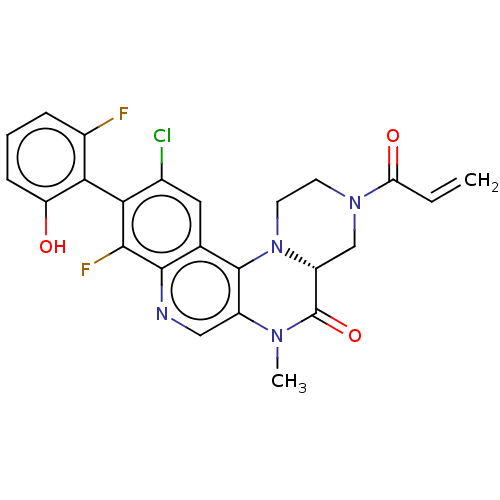
(CHEMBL4452974)Show SMILES [H][C@]12CN(CCN1c1c(cnc3c(F)c(c(Cl)cc13)-c1c(O)cccc1F)N(C)C2=O)C(=O)C=C |r,wU:1.0,(30.86,-26.9,;29.54,-27.68,;29.53,-26.14,;28.19,-25.38,;26.86,-26.15,;26.87,-27.69,;28.21,-28.46,;28.22,-30,;29.55,-30.76,;29.56,-32.31,;28.23,-33.08,;26.89,-32.31,;25.56,-33.09,;25.56,-34.63,;24.22,-32.32,;24.22,-30.78,;22.89,-30.01,;25.55,-30.01,;26.89,-30.77,;22.89,-33.09,;21.56,-32.32,;21.56,-30.78,;20.22,-33.08,;20.22,-34.62,;21.57,-35.4,;22.89,-34.62,;24.23,-35.39,;30.88,-29.98,;32.22,-30.74,;30.87,-28.44,;32.2,-27.66,;28.18,-23.84,;26.84,-23.07,;29.51,-23.06,;29.5,-21.52,)| Show InChI InChI=1S/C24H19ClF2N4O3/c1-3-18(33)30-7-8-31-16(11-30)24(34)29(2)15-10-28-22-12(23(15)31)9-13(25)19(21(22)27)20-14(26)5-4-6-17(20)32/h3-6,9-10,16,32H,1,7-8,11H2,2H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50521249
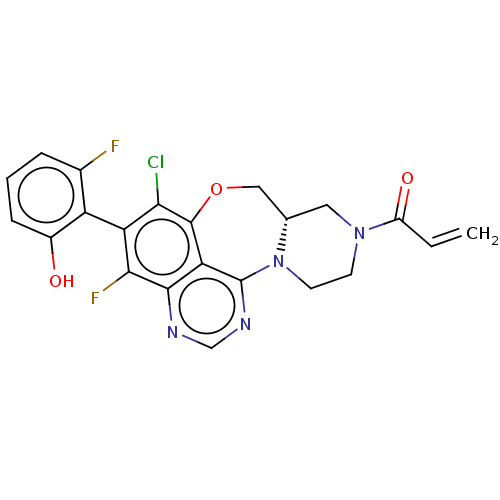
(CHEMBL4452819)Show SMILES [H][C@@]12CN(CCN1c1ncnc3c(F)c(c(Cl)c(OC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(15.59,-28.29,;14.81,-29.62,;16.34,-29.49,;17.21,-30.76,;16.55,-32.15,;15.02,-32.27,;14.16,-31.01,;12.73,-31.25,;12.79,-32.74,;11.44,-33.52,;10.11,-32.74,;10.11,-31.19,;8.78,-30.44,;7.45,-31.21,;8.77,-28.91,;10.1,-28.13,;10.1,-26.58,;11.43,-28.89,;12.63,-27.92,;14.14,-28.24,;11.45,-30.43,;7.43,-28.14,;6.1,-28.93,;6.12,-30.46,;4.76,-28.17,;4.74,-26.61,;6.08,-25.83,;7.42,-26.6,;8.76,-25.82,;18.75,-30.64,;19.42,-29.24,;19.63,-31.91,;21.17,-31.78,)| Show InChI InChI=1S/C22H17ClF2N4O3/c1-2-14(31)28-6-7-29-11(8-28)9-32-21-17-20(26-10-27-22(17)29)19(25)16(18(21)23)15-12(24)4-3-5-13(15)30/h2-5,10-11,30H,1,6-9H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50185140
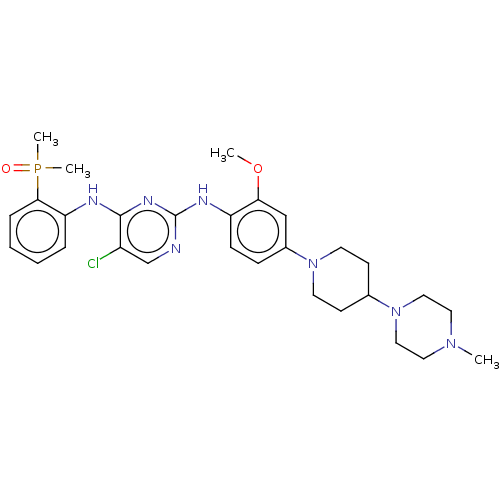
(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Wild type EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50580190

(CHEMBL5087264)Show SMILES COc1cc(N2CCOCC2)c(cc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)-c1cnn(C)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR L858R/T790M mutant (unknown origin) expressed in human NCI-H1975 cells assessed as protein phosphorylation measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527058
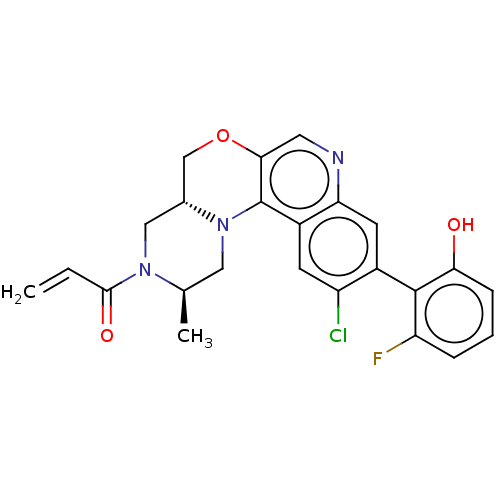
(CHEMBL4455191)Show SMILES [H][C@@]12COc3cnc4cc(c(Cl)cc4c3N1C[C@@H](C)N(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,17.20,(70.37,-6.5,;69.05,-7.29,;70.38,-8.05,;70.39,-9.59,;69.06,-10.36,;69.07,-11.91,;67.74,-12.69,;66.4,-11.92,;65.07,-12.7,;63.73,-11.93,;63.73,-10.38,;62.4,-9.61,;65.06,-9.61,;66.4,-10.38,;67.72,-9.6,;67.72,-8.06,;66.38,-7.3,;66.37,-5.76,;65.03,-5,;67.7,-4.98,;69.04,-5.75,;67.69,-3.44,;66.35,-2.68,;69.02,-2.67,;69.01,-1.13,;62.4,-12.7,;61.07,-11.92,;61.07,-10.38,;59.73,-12.69,;59.73,-14.23,;61.08,-15,;62.4,-14.23,;63.74,-15,)| Show InChI InChI=1S/C24H21ClFN3O3/c1-3-22(31)28-11-14-12-32-21-9-27-19-8-15(23-18(26)5-4-6-20(23)30)17(25)7-16(19)24(21)29(14)10-13(28)2/h3-9,13-14,30H,1,10-12H2,2H3/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527050
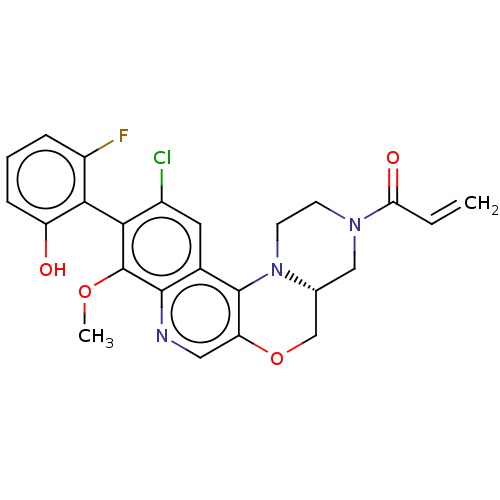
(CHEMBL4572730)Show SMILES [H][C@@]12COc3cnc4c(OC)c(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(51.05,-48.22,;49.73,-49.01,;51.06,-49.76,;51.07,-51.3,;49.74,-52.08,;49.75,-53.63,;48.42,-54.41,;47.08,-53.64,;45.75,-54.42,;45.75,-55.96,;47.09,-56.72,;44.41,-53.65,;44.42,-52.1,;43.08,-51.33,;45.74,-51.33,;47.08,-52.1,;48.41,-51.32,;48.4,-49.78,;47.06,-49.02,;47.05,-47.48,;48.38,-46.7,;49.72,-47.47,;48.37,-45.16,;47.04,-44.4,;49.7,-44.39,;49.69,-42.85,;43.08,-54.42,;41.75,-53.64,;41.75,-52.1,;40.42,-54.41,;40.41,-55.95,;41.76,-56.72,;43.08,-55.95,;44.42,-56.72,)| Show InChI InChI=1S/C24H21ClFN3O4/c1-3-19(31)28-7-8-29-13(11-28)12-33-18-10-27-22-14(23(18)29)9-15(25)20(24(22)32-2)21-16(26)5-4-6-17(21)30/h3-6,9-10,13,30H,1,7-8,11-12H2,2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527054
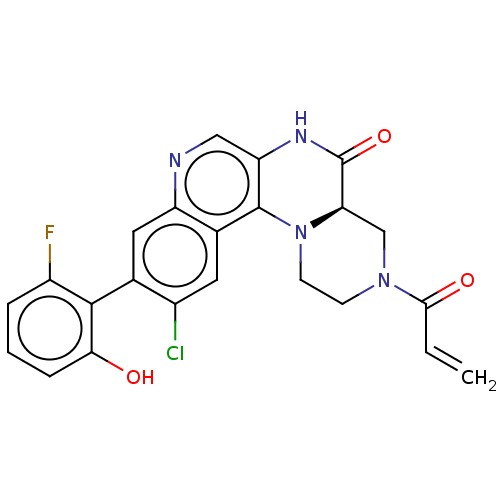
(CHEMBL4453777)Show SMILES [H][C@]12CN(CCN1c1c(NC2=O)cnc2cc(c(Cl)cc12)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,(31.71,-6.52,;30.38,-7.3,;30.38,-5.76,;29.04,-5,;27.71,-5.78,;27.72,-7.32,;29.05,-8.08,;29.06,-9.62,;30.4,-10.38,;31.73,-9.6,;31.72,-8.06,;33.05,-7.28,;30.41,-11.93,;29.07,-12.7,;27.74,-11.94,;26.4,-12.71,;25.07,-11.94,;25.07,-10.4,;23.74,-9.63,;26.4,-9.63,;27.74,-10.39,;23.74,-12.71,;22.41,-11.94,;22.41,-10.4,;21.07,-12.71,;21.07,-14.25,;22.41,-15.02,;23.74,-14.25,;25.08,-15.01,;29.03,-3.46,;27.69,-2.7,;30.36,-2.68,;30.35,-1.14,)| Show InChI InChI=1S/C23H18ClFN4O3/c1-2-20(31)28-6-7-29-18(11-28)23(32)27-17-10-26-16-9-12(14(24)8-13(16)22(17)29)21-15(25)4-3-5-19(21)30/h2-5,8-10,18,30H,1,6-7,11H2,(H,27,32)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50521250
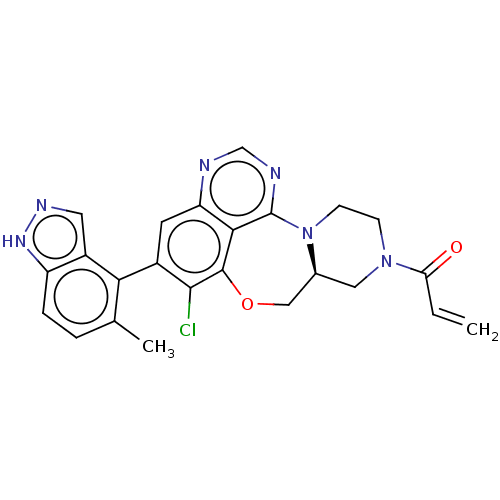
(CHEMBL4475526)Show SMILES [H][C@@]12CN(CCN1c1ncnc3cc(c(Cl)c(OC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wU:1.0,(15.23,-3.53,;14.46,-4.86,;15.98,-4.73,;16.85,-6,;16.19,-7.38,;14.67,-7.5,;13.8,-6.25,;12.38,-6.49,;12.43,-7.97,;11.09,-8.75,;9.76,-7.97,;9.76,-6.43,;8.43,-5.67,;8.42,-4.15,;9.75,-3.37,;9.75,-1.82,;11.08,-4.13,;12.28,-3.16,;13.78,-3.48,;11.1,-5.66,;7.08,-3.38,;7.08,-1.84,;8.41,-1.06,;5.74,-1.08,;4.4,-1.86,;4.42,-3.41,;3.28,-4.45,;3.92,-5.85,;5.46,-5.68,;5.76,-4.17,;18.39,-5.87,;19.05,-4.48,;19.27,-7.14,;20.8,-7.02,)| Show InChI InChI=1S/C24H21ClN6O2/c1-3-19(32)30-6-7-31-14(10-30)11-33-23-21-18(26-12-27-24(21)31)8-15(22(23)25)20-13(2)4-5-17-16(20)9-28-29-17/h3-5,8-9,12,14H,1,6-7,10-11H2,2H3,(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50521250

(CHEMBL4475526)Show SMILES [H][C@@]12CN(CCN1c1ncnc3cc(c(Cl)c(OC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wU:1.0,(15.23,-3.53,;14.46,-4.86,;15.98,-4.73,;16.85,-6,;16.19,-7.38,;14.67,-7.5,;13.8,-6.25,;12.38,-6.49,;12.43,-7.97,;11.09,-8.75,;9.76,-7.97,;9.76,-6.43,;8.43,-5.67,;8.42,-4.15,;9.75,-3.37,;9.75,-1.82,;11.08,-4.13,;12.28,-3.16,;13.78,-3.48,;11.1,-5.66,;7.08,-3.38,;7.08,-1.84,;8.41,-1.06,;5.74,-1.08,;4.4,-1.86,;4.42,-3.41,;3.28,-4.45,;3.92,-5.85,;5.46,-5.68,;5.76,-4.17,;18.39,-5.87,;19.05,-4.48,;19.27,-7.14,;20.8,-7.02,)| Show InChI InChI=1S/C24H21ClN6O2/c1-3-19(32)30-6-7-31-14(10-30)11-33-23-21-18(26-12-27-24(21)31)8-15(22(23)25)20-13(2)4-5-17-16(20)9-28-29-17/h3-5,8-9,12,14H,1,6-7,10-11H2,2H3,(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527046
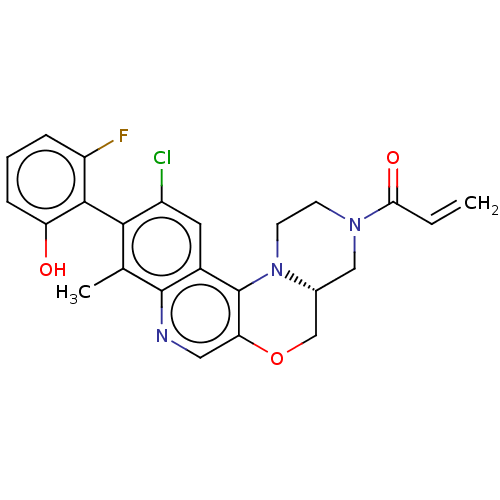
(CHEMBL4567284)Show SMILES [H][C@@]12COc3cnc4c(C)c(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(30.95,-48.11,;29.62,-48.9,;30.96,-49.66,;30.97,-51.2,;29.64,-51.97,;29.65,-53.52,;28.31,-54.3,;26.98,-53.53,;25.64,-54.31,;25.65,-55.85,;24.31,-53.54,;24.31,-51.99,;22.98,-51.22,;25.64,-51.22,;26.97,-51.99,;28.3,-51.21,;28.29,-49.67,;26.95,-48.91,;26.95,-47.37,;28.28,-46.59,;29.61,-47.36,;28.27,-45.05,;26.93,-44.29,;29.6,-44.28,;29.59,-42.74,;22.98,-54.31,;21.65,-53.53,;21.65,-51.99,;20.31,-54.3,;20.31,-55.84,;21.65,-56.61,;22.98,-55.84,;24.31,-56.61,)| Show InChI InChI=1S/C24H21ClFN3O3/c1-3-20(31)28-7-8-29-14(11-28)12-32-19-10-27-23-13(2)21(16(25)9-15(23)24(19)29)22-17(26)5-4-6-18(22)30/h3-6,9-10,14,30H,1,7-8,11-12H2,2H3/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605541
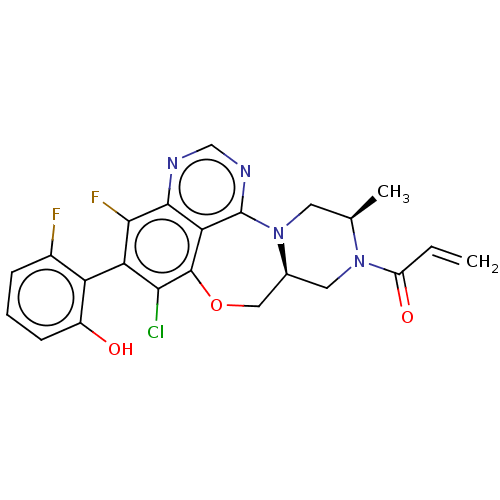
(CHEMBL5192093)Show SMILES [H][C@@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,wD:4.4,(2.72,2.83,;2.65,1.09,;4,1.85,;5.32,1.07,;5.31,-.47,;6.64,-1.24,;3.97,-1.23,;2.64,-.45,;1.42,-1.39,;2.02,-2.78,;1.05,-3.98,;-.47,-3.73,;-.95,-2.3,;-2.49,-2.17,;-3.19,-3.18,;-3.14,-.78,;-2.26,.49,;-2.79,1.6,;-.73,.36,;-.05,1.74,;1.46,2.07,;-.07,-1.04,;-4.68,-.65,;-5.56,-1.91,;-5.04,-3.03,;-7.09,-1.78,;-7.75,-.38,;-6.86,.88,;-5.33,.75,;-4.62,1.76,;6.66,1.83,;7.72,1.2,;6.68,3.37,;7.75,3.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605541

(CHEMBL5192093)Show SMILES [H][C@@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,wD:4.4,(2.72,2.83,;2.65,1.09,;4,1.85,;5.32,1.07,;5.31,-.47,;6.64,-1.24,;3.97,-1.23,;2.64,-.45,;1.42,-1.39,;2.02,-2.78,;1.05,-3.98,;-.47,-3.73,;-.95,-2.3,;-2.49,-2.17,;-3.19,-3.18,;-3.14,-.78,;-2.26,.49,;-2.79,1.6,;-.73,.36,;-.05,1.74,;1.46,2.07,;-.07,-1.04,;-4.68,-.65,;-5.56,-1.91,;-5.04,-3.03,;-7.09,-1.78,;-7.75,-.38,;-6.86,.88,;-5.33,.75,;-4.62,1.76,;6.66,1.83,;7.72,1.2,;6.68,3.37,;7.75,3.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527055

(CHEMBL4461913)Show SMILES [H][C@@]12CN(C)c3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(13.02,-6.95,;11.69,-7.74,;13.03,-8.5,;13.04,-10.04,;14.38,-10.8,;11.71,-10.81,;11.72,-12.36,;10.38,-13.14,;9.05,-12.37,;7.71,-13.15,;6.38,-12.38,;6.38,-10.83,;5.05,-10.06,;7.71,-10.06,;9.05,-10.83,;10.37,-10.05,;10.36,-8.51,;9.03,-7.75,;9.02,-6.21,;10.35,-5.43,;11.69,-6.2,;10.34,-3.89,;9,-3.13,;11.67,-3.12,;11.66,-1.58,;5.05,-13.15,;3.72,-12.37,;3.72,-10.83,;2.38,-13.14,;2.38,-14.68,;3.72,-15.45,;5.05,-14.68,;6.39,-15.45,)| Show InChI InChI=1S/C24H22ClFN4O2/c1-3-22(32)29-7-8-30-14(13-29)12-28(2)20-11-27-19-10-15(17(25)9-16(19)24(20)30)23-18(26)5-4-6-21(23)31/h3-6,9-11,14,31H,1,7-8,12-13H2,2H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605542
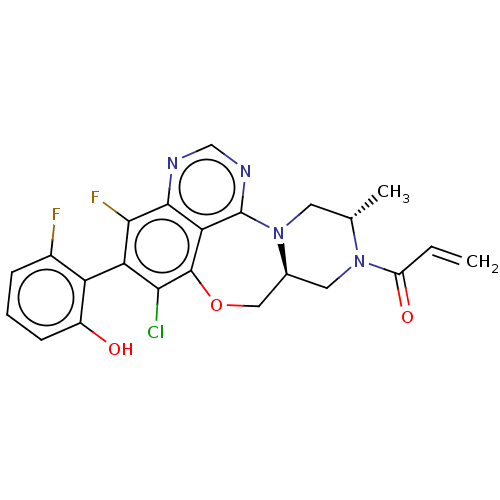
(CHEMBL5190874)Show SMILES [H][C@@]12CN([C@@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wU:1.0,4.4,(2.72,2.83,;2.65,1.09,;4,1.85,;5.32,1.07,;5.31,-.47,;6.64,-1.24,;3.97,-1.23,;2.64,-.45,;1.42,-1.39,;2.02,-2.78,;1.05,-3.98,;-.47,-3.73,;-.95,-2.3,;-2.49,-2.17,;-3.19,-3.18,;-3.14,-.78,;-2.26,.49,;-2.79,1.6,;-.73,.36,;-.05,1.74,;1.46,2.07,;-.07,-1.04,;-4.68,-.65,;-5.56,-1.91,;-5.04,-3.03,;-7.09,-1.78,;-7.75,-.38,;-6.86,.88,;-5.33,.75,;-4.62,1.76,;6.66,1.83,;7.72,1.2,;6.68,3.37,;7.75,3.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605544
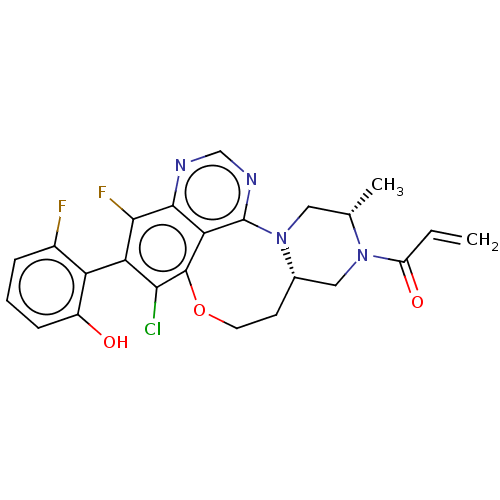
(CHEMBL5173371)Show SMILES [H][C@]12CN([C@@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wU:4.4,wD:1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;5.94,-1.59,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527048
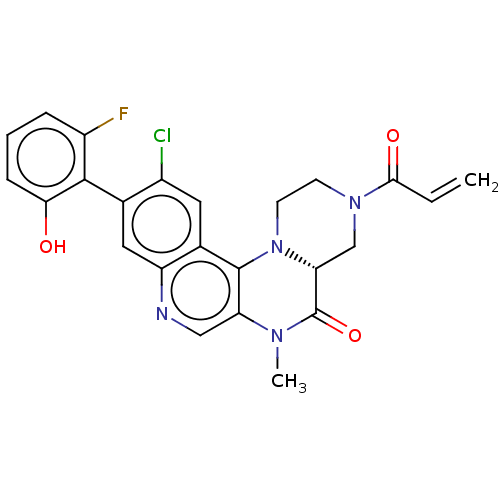
(CHEMBL4551121)Show SMILES [H][C@]12CN(CCN1c1c(cnc3cc(c(Cl)cc13)-c1c(O)cccc1F)N(C)C2=O)C(=O)C=C |r,wU:1.0,(50.13,-6.75,;48.8,-7.54,;48.79,-6,;47.46,-5.23,;46.13,-6.01,;46.13,-7.55,;47.47,-8.31,;47.48,-9.85,;48.82,-10.61,;48.83,-12.16,;47.49,-12.94,;46.16,-12.17,;44.82,-12.95,;43.49,-12.18,;43.49,-10.63,;42.16,-9.86,;44.82,-9.86,;46.15,-10.63,;42.16,-12.95,;40.83,-12.17,;40.83,-10.63,;39.49,-12.94,;39.49,-14.48,;40.83,-15.25,;42.16,-14.48,;43.49,-15.25,;50.15,-9.83,;51.48,-10.6,;50.14,-8.29,;51.47,-7.52,;47.45,-3.69,;46.11,-2.93,;48.78,-2.92,;48.77,-1.38,)| Show InChI InChI=1S/C24H20ClFN4O3/c1-3-21(32)29-7-8-30-19(12-29)24(33)28(2)18-11-27-17-10-13(15(25)9-14(17)23(18)30)22-16(26)5-4-6-20(22)31/h3-6,9-11,19,31H,1,7-8,12H2,2H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605538
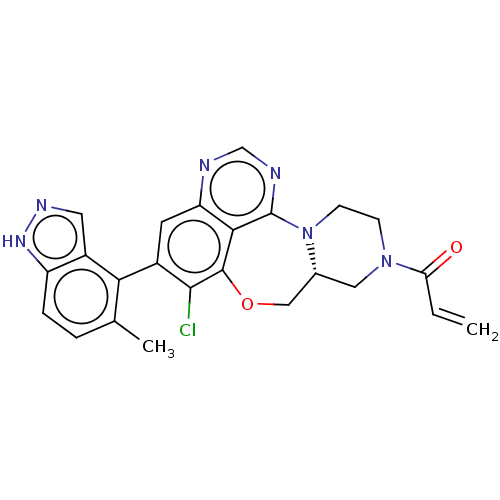
(CHEMBL5185314)Show SMILES [H][C@]12CN(CCN1c1ncnc3cc(c(Cl)c(OC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wD:1.0,(.69,4.05,;1.46,2.71,;2.18,4.1,;3.71,4.1,;4.53,2.82,;3.76,1.49,;2.23,1.43,;1.72,-.05,;2.95,-.87,;2.79,-2.41,;1.36,-3.02,;.18,-2.05,;-1.15,-2.77,;-2.48,-1.95,;-2.38,-.41,;-3.41,.26,;-1.05,.31,;-1.2,1.84,;-.08,2.92,;.28,-.51,;-3.82,-2.61,;-5.1,-1.79,;-5.05,-.56,;-6.48,-2.51,;-6.58,-4.05,;-5.25,-4.87,;-5.05,-6.4,;-3.51,-6.61,;-2.79,-5.28,;-3.92,-4.15,;4.43,5.48,;3.82,6.51,;5.97,5.53,;6.58,6.61,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605538

(CHEMBL5185314)Show SMILES [H][C@]12CN(CCN1c1ncnc3cc(c(Cl)c(OC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wD:1.0,(.69,4.05,;1.46,2.71,;2.18,4.1,;3.71,4.1,;4.53,2.82,;3.76,1.49,;2.23,1.43,;1.72,-.05,;2.95,-.87,;2.79,-2.41,;1.36,-3.02,;.18,-2.05,;-1.15,-2.77,;-2.48,-1.95,;-2.38,-.41,;-3.41,.26,;-1.05,.31,;-1.2,1.84,;-.08,2.92,;.28,-.51,;-3.82,-2.61,;-5.1,-1.79,;-5.05,-.56,;-6.48,-2.51,;-6.58,-4.05,;-5.25,-4.87,;-5.05,-6.4,;-3.51,-6.61,;-2.79,-5.28,;-3.92,-4.15,;4.43,5.48,;3.82,6.51,;5.97,5.53,;6.58,6.61,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50185140

(AP-26113 | Brigatinib | US11248003, Example Brigat...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C29H39ClN7O2P/c1-35-15-17-37(18-16-35)21-11-13-36(14-12-21)22-9-10-24(26(19-22)39-2)33-29-31-20-23(30)28(34-29)32-25-7-5-6-8-27(25)40(3,4)38/h5-10,19-21H,11-18H2,1-4H3,(H2,31,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR L858R/T790M mutant (unknown origin) expressed in human NCI-H1975 cells assessed as protein phosphorylation measured after 2 hrs by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01055
BindingDB Entry DOI: 10.7270/Q2959NF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527061
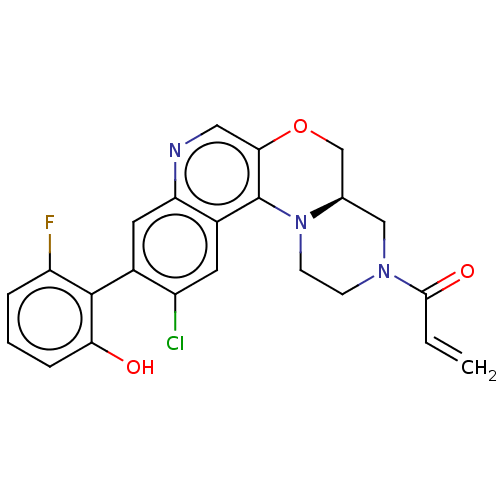
(CHEMBL4456368)Show SMILES [H][C@@]12COc3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(71.28,-28.06,;69.95,-28.85,;71.29,-29.61,;71.3,-31.15,;69.97,-31.92,;69.98,-33.47,;68.64,-34.25,;67.31,-33.48,;65.97,-34.26,;64.64,-33.49,;64.64,-31.94,;63.31,-31.17,;65.97,-31.17,;67.3,-31.94,;68.63,-31.16,;68.62,-29.62,;67.28,-28.86,;67.28,-27.32,;68.61,-26.54,;69.94,-27.31,;68.6,-25,;67.26,-24.24,;69.93,-24.23,;69.92,-22.69,;63.31,-34.26,;61.97,-33.48,;61.98,-31.94,;60.64,-34.25,;60.64,-35.79,;61.98,-36.56,;63.31,-35.79,;64.64,-36.56,)| Show InChI InChI=1S/C23H19ClFN3O3/c1-2-21(30)27-6-7-28-13(11-27)12-31-20-10-26-18-9-14(16(24)8-15(18)23(20)28)22-17(25)4-3-5-19(22)29/h2-5,8-10,13,29H,1,6-7,11-12H2/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527047
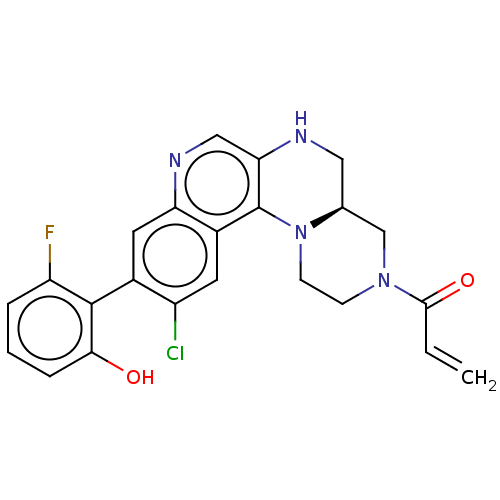
(CHEMBL4560503)Show SMILES [H][C@@]12CNc3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,(70.81,-47.87,;69.48,-48.66,;70.82,-49.41,;70.83,-50.95,;69.5,-51.73,;69.51,-53.28,;68.17,-54.06,;66.84,-53.29,;65.5,-54.07,;64.17,-53.3,;64.17,-51.75,;62.84,-50.98,;65.5,-50.98,;66.84,-51.75,;68.16,-50.97,;68.15,-49.43,;66.82,-48.67,;66.81,-47.13,;68.14,-46.35,;69.47,-47.12,;68.13,-44.81,;66.79,-44.05,;69.46,-44.04,;69.45,-42.5,;62.84,-54.07,;61.51,-53.29,;61.51,-51.75,;60.17,-54.06,;60.17,-55.6,;61.51,-56.37,;62.84,-55.6,;64.17,-56.37,)| Show InChI InChI=1S/C23H20ClFN4O2/c1-2-21(31)28-6-7-29-13(12-28)10-26-19-11-27-18-9-14(16(24)8-15(18)23(19)29)22-17(25)4-3-5-20(22)30/h2-5,8-9,11,13,26,30H,1,6-7,10,12H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527056
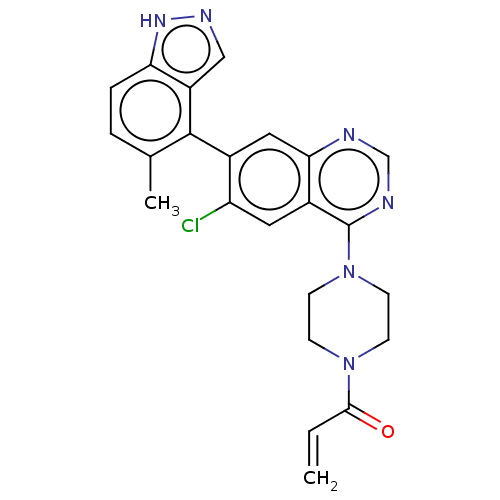
(CHEMBL4536127)Show SMILES Cc1ccc2[nH]ncc2c1-c1cc2ncnc(N3CCN(CC3)C(=O)C=C)c2cc1Cl |(9.04,-15.37,;7.7,-14.6,;6.38,-15.38,;5.03,-14.61,;5.04,-13.06,;3.89,-12.03,;4.52,-10.63,;6.05,-10.79,;6.37,-12.3,;7.7,-13.07,;9.03,-12.3,;10.37,-13.07,;11.7,-12.29,;13.04,-13.06,;14.37,-12.29,;14.36,-10.74,;13.03,-9.98,;13.02,-8.44,;11.68,-7.67,;11.67,-6.13,;13,-5.36,;14.34,-6.12,;14.35,-7.66,;12.99,-3.82,;11.65,-3.05,;14.32,-3.04,;14.31,-1.5,;11.7,-10.75,;10.36,-9.99,;9.03,-10.76,;7.7,-9.99,)| Show InChI InChI=1S/C23H21ClN6O/c1-3-21(31)29-6-8-30(9-7-29)23-16-10-18(24)15(11-20(16)25-13-26-23)22-14(2)4-5-19-17(22)12-27-28-19/h3-5,10-13H,1,6-9H2,2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527056

(CHEMBL4536127)Show SMILES Cc1ccc2[nH]ncc2c1-c1cc2ncnc(N3CCN(CC3)C(=O)C=C)c2cc1Cl |(9.04,-15.37,;7.7,-14.6,;6.38,-15.38,;5.03,-14.61,;5.04,-13.06,;3.89,-12.03,;4.52,-10.63,;6.05,-10.79,;6.37,-12.3,;7.7,-13.07,;9.03,-12.3,;10.37,-13.07,;11.7,-12.29,;13.04,-13.06,;14.37,-12.29,;14.36,-10.74,;13.03,-9.98,;13.02,-8.44,;11.68,-7.67,;11.67,-6.13,;13,-5.36,;14.34,-6.12,;14.35,-7.66,;12.99,-3.82,;11.65,-3.05,;14.32,-3.04,;14.31,-1.5,;11.7,-10.75,;10.36,-9.99,;9.03,-10.76,;7.7,-9.99,)| Show InChI InChI=1S/C23H21ClN6O/c1-3-21(31)29-6-8-30(9-7-29)23-16-10-18(24)15(11-20(16)25-13-26-23)22-14(2)4-5-19-17(22)12-27-28-19/h3-5,10-13H,1,6-9H2,2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527053
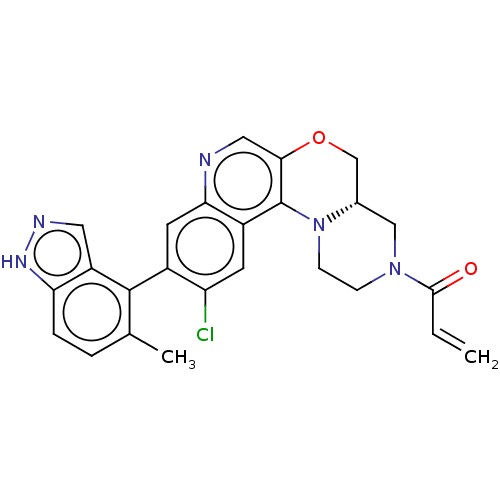
(CHEMBL4454588)Show SMILES [H][C@]12COc3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(C)ccc2[nH]ncc12 |r,wD:1.0,(33.86,-6.64,;32.53,-7.43,;33.87,-8.19,;33.87,-9.73,;32.55,-10.5,;32.56,-12.05,;31.22,-12.83,;29.89,-12.06,;28.55,-12.84,;27.22,-12.07,;27.22,-10.52,;25.89,-9.75,;28.55,-9.75,;29.88,-10.52,;31.21,-9.74,;31.2,-8.2,;29.86,-7.44,;29.86,-5.9,;31.19,-5.12,;32.52,-5.89,;31.18,-3.58,;29.84,-2.82,;32.51,-2.81,;32.5,-1.27,;25.89,-12.84,;25.89,-14.37,;27.22,-15.14,;24.56,-15.14,;23.22,-14.37,;23.22,-12.83,;22.08,-11.8,;22.71,-10.39,;24.24,-10.56,;24.55,-12.06,)| Show InChI InChI=1S/C25H22ClN5O2/c1-3-23(32)30-6-7-31-15(12-30)13-33-22-11-27-21-9-16(19(26)8-17(21)25(22)31)24-14(2)4-5-20-18(24)10-28-29-20/h3-5,8-11,15H,1,6-7,12-13H2,2H3,(H,28,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527053

(CHEMBL4454588)Show SMILES [H][C@]12COc3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(C)ccc2[nH]ncc12 |r,wD:1.0,(33.86,-6.64,;32.53,-7.43,;33.87,-8.19,;33.87,-9.73,;32.55,-10.5,;32.56,-12.05,;31.22,-12.83,;29.89,-12.06,;28.55,-12.84,;27.22,-12.07,;27.22,-10.52,;25.89,-9.75,;28.55,-9.75,;29.88,-10.52,;31.21,-9.74,;31.2,-8.2,;29.86,-7.44,;29.86,-5.9,;31.19,-5.12,;32.52,-5.89,;31.18,-3.58,;29.84,-2.82,;32.51,-2.81,;32.5,-1.27,;25.89,-12.84,;25.89,-14.37,;27.22,-15.14,;24.56,-15.14,;23.22,-14.37,;23.22,-12.83,;22.08,-11.8,;22.71,-10.39,;24.24,-10.56,;24.55,-12.06,)| Show InChI InChI=1S/C25H22ClN5O2/c1-3-23(32)30-6-7-31-15(12-30)13-33-22-11-27-21-9-16(19(26)8-17(21)25(22)31)24-14(2)4-5-20-18(24)10-28-29-20/h3-5,8-11,15H,1,6-7,12-13H2,2H3,(H,28,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527049
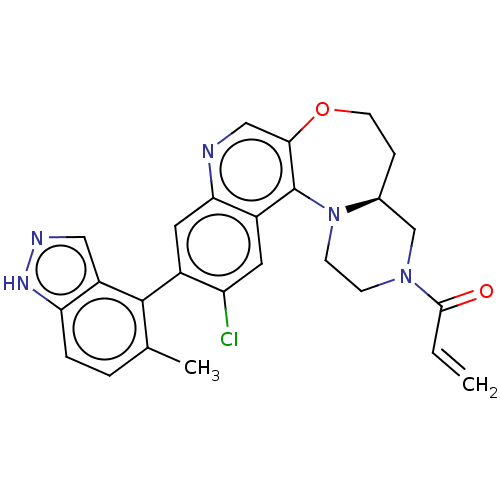
(CHEMBL4548586)Show SMILES [H][C@]12CN(CCN1c1c(OCC2)cnc2cc(c(Cl)cc12)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wU:1.0,(51.2,-26.9,;50.11,-28,;49.65,-26.54,;48.14,-26.2,;47.09,-27.35,;47.56,-28.83,;49.07,-29.15,;49.31,-30.68,;50.64,-31.45,;52.09,-30.88,;52.54,-29.39,;51.65,-28.12,;50.65,-32.99,;49.32,-33.76,;47.99,-32.99,;46.65,-33.77,;45.32,-33,;45.32,-31.46,;43.99,-30.69,;46.65,-30.69,;47.98,-31.45,;43.99,-33.77,;43.99,-35.3,;45.33,-36.07,;42.66,-36.07,;41.32,-35.3,;41.32,-33.76,;40.18,-32.73,;40.81,-31.33,;42.34,-31.49,;42.66,-33,;47.67,-24.73,;46.17,-24.4,;48.71,-23.6,;48.24,-22.13,)| Show InChI InChI=1S/C26H24ClN5O2/c1-3-24(33)31-7-8-32-16(14-31)6-9-34-23-13-28-22-11-17(20(27)10-18(22)26(23)32)25-15(2)4-5-21-19(25)12-29-30-21/h3-5,10-13,16H,1,6-9,14H2,2H3,(H,29,30)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 627 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527052

(CHEMBL4483782)Show SMILES [H][C@@]12COc3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(C)ccc2[nH]ncc12 |r,wU:1.0,(71.97,-6.9,;70.65,-7.68,;71.98,-8.44,;71.99,-9.98,;70.66,-10.76,;70.67,-12.31,;69.34,-13.08,;68,-12.32,;66.67,-13.09,;65.33,-12.32,;65.34,-10.78,;64,-10.01,;66.67,-10.01,;68,-10.77,;69.33,-10,;69.32,-8.46,;67.98,-7.7,;67.97,-6.16,;69.3,-5.38,;70.64,-6.14,;69.29,-3.84,;67.96,-3.08,;70.62,-3.06,;70.62,-1.52,;64,-13.09,;64.01,-14.63,;65.34,-15.39,;62.68,-15.4,;61.33,-14.63,;61.34,-13.09,;60.19,-12.05,;60.82,-10.65,;62.35,-10.81,;62.67,-12.32,)| Show InChI InChI=1S/C25H22ClN5O2/c1-3-23(32)30-6-7-31-15(12-30)13-33-22-11-27-21-9-16(19(26)8-17(21)25(22)31)24-14(2)4-5-20-18(24)10-28-29-20/h3-5,8-11,15H,1,6-7,12-13H2,2H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527052

(CHEMBL4483782)Show SMILES [H][C@@]12COc3cnc4cc(c(Cl)cc4c3N1CCN(C2)C(=O)C=C)-c1c(C)ccc2[nH]ncc12 |r,wU:1.0,(71.97,-6.9,;70.65,-7.68,;71.98,-8.44,;71.99,-9.98,;70.66,-10.76,;70.67,-12.31,;69.34,-13.08,;68,-12.32,;66.67,-13.09,;65.33,-12.32,;65.34,-10.78,;64,-10.01,;66.67,-10.01,;68,-10.77,;69.33,-10,;69.32,-8.46,;67.98,-7.7,;67.97,-6.16,;69.3,-5.38,;70.64,-6.14,;69.29,-3.84,;67.96,-3.08,;70.62,-3.06,;70.62,-1.52,;64,-13.09,;64.01,-14.63,;65.34,-15.39,;62.68,-15.4,;61.33,-14.63,;61.34,-13.09,;60.19,-12.05,;60.82,-10.65,;62.35,-10.81,;62.67,-12.32,)| Show InChI InChI=1S/C25H22ClN5O2/c1-3-23(32)30-6-7-31-15(12-30)13-33-22-11-27-21-9-16(19(26)8-17(21)25(22)31)24-14(2)4-5-20-18(24)10-28-29-20/h3-5,8-11,15H,1,6-7,12-13H2,2H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527062
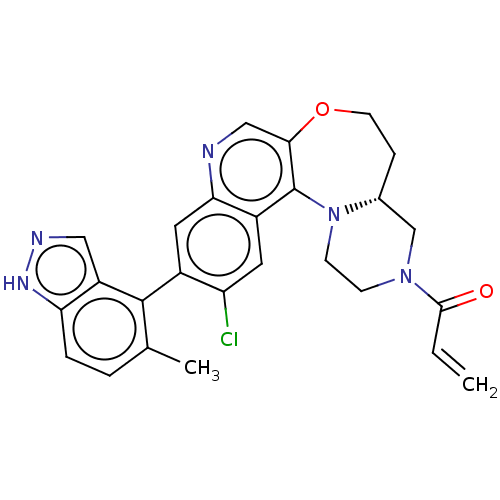
(CHEMBL4456612)Show SMILES [H][C@@]12CN(CCN1c1c(OCC2)cnc2cc(c(Cl)cc12)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wD:1.0,(31.76,-26.84,;30.67,-27.95,;30.2,-26.48,;28.69,-26.14,;27.65,-27.29,;28.11,-28.77,;29.63,-29.09,;29.87,-30.62,;31.2,-31.39,;32.64,-30.82,;33.09,-29.33,;32.21,-28.06,;31.21,-32.93,;29.88,-33.7,;28.54,-32.93,;27.21,-33.71,;25.87,-32.94,;25.88,-31.4,;24.54,-30.63,;27.2,-30.63,;28.54,-31.39,;24.54,-33.71,;24.55,-35.24,;25.88,-36.01,;23.22,-36.02,;21.88,-35.25,;21.88,-33.7,;20.74,-32.67,;21.36,-31.27,;22.9,-31.43,;23.21,-32.94,;28.23,-24.67,;26.72,-24.34,;29.26,-23.54,;28.8,-22.07,)| Show InChI InChI=1S/C26H24ClN5O2/c1-3-24(33)31-7-8-32-16(14-31)6-9-34-23-13-28-22-11-17(20(27)10-18(22)26(23)32)25-15(2)4-5-21-19(25)12-29-30-21/h3-5,10-13,16H,1,6-9,14H2,2H3,(H,29,30)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50527060
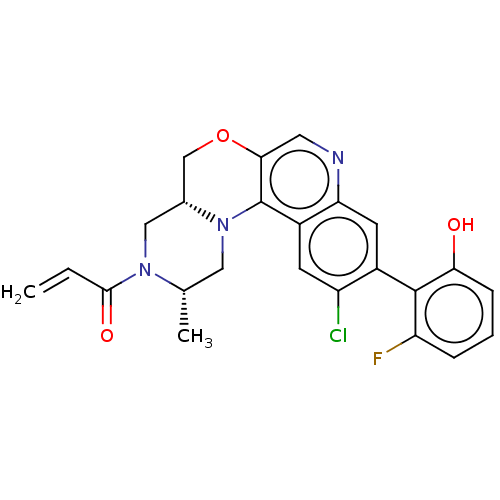
(CHEMBL4461958)Show SMILES [H][C@@]12COc3cnc4cc(c(Cl)cc4c3N1C[C@H](C)N(C2)C(=O)C=C)-c1c(O)cccc1F |r,wU:1.0,wD:17.20,(12.87,-26.71,;11.55,-27.49,;12.88,-28.25,;12.89,-29.79,;11.56,-30.57,;11.57,-32.12,;10.24,-32.89,;8.9,-32.13,;7.57,-32.9,;6.23,-32.13,;6.23,-30.59,;4.9,-29.82,;7.56,-29.82,;8.9,-30.58,;10.23,-29.81,;10.22,-28.27,;8.88,-27.51,;8.87,-25.97,;7.53,-25.2,;10.2,-25.19,;11.54,-25.95,;10.19,-23.65,;8.85,-22.89,;11.52,-22.87,;11.51,-21.33,;4.9,-32.9,;3.57,-32.13,;3.57,-30.59,;2.24,-32.9,;2.23,-34.44,;3.58,-35.21,;4.9,-34.44,;6.24,-35.2,)| Show InChI InChI=1S/C24H21ClFN3O3/c1-3-22(31)28-11-14-12-32-21-9-27-19-8-15(23-18(26)5-4-6-20(23)30)17(25)7-16(19)24(21)29(14)10-13(28)2/h3-9,13-14,30H,1,10-12H2,2H3/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... |
J Med Chem 63: 4468-4483 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01720
BindingDB Entry DOI: 10.7270/Q2F76H10 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605539
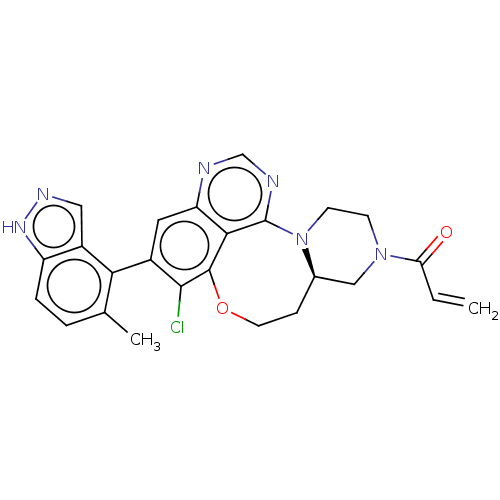
(CHEMBL5172544)Show SMILES [H][C@@]12CN(CCN1c1ncnc3cc(c(Cl)c(OCC2)c13)-c1c(C)ccc2[nH]ncc12)C(=O)C=C |r,wU:1.0,(.27,4.4,;1.04,3.07,;1.88,4.35,;3.42,4.26,;4.11,2.89,;3.27,1.6,;1.73,1.69,;1.3,.21,;2.65,-.54,;2.67,-2.08,;1.35,-2.87,;0,-2.12,;-1.32,-2.91,;-2.66,-2.17,;-2.69,-.63,;-4.03,.12,;-1.37,.17,;-1.94,1.5,;-1.83,3.02,;-.41,3.6,;-.02,-.58,;-3.98,-2.96,;-5.33,-2.21,;-5.36,-.67,;-6.65,-3,;-6.62,-4.54,;-5.28,-5.29,;-4.93,-6.79,;-3.4,-6.93,;-2.8,-5.51,;-3.96,-4.5,;4.27,5.55,;3.58,6.93,;5.8,5.46,;6.65,6.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data