

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509708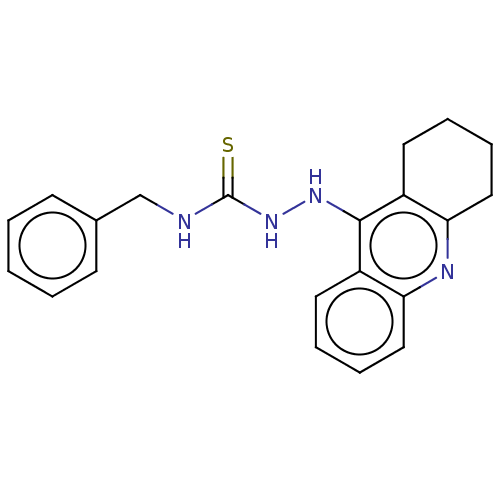 (CHEMBL4458648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human AChE assessed as dissociation constant using acetylthiocholine iodide as substrate incubated for 10 mins b... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509708 (CHEMBL4458648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human AChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using acetylthiocholine iodi... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509709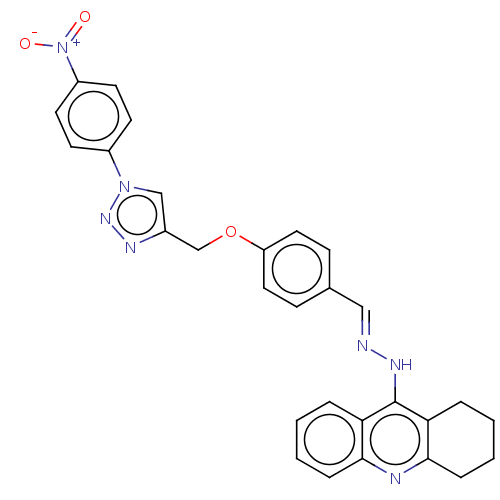 (CHEMBL4441059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509708 (CHEMBL4458648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509718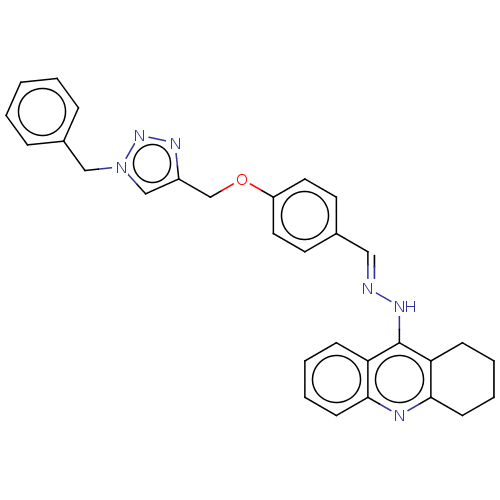 (CHEMBL4466829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509705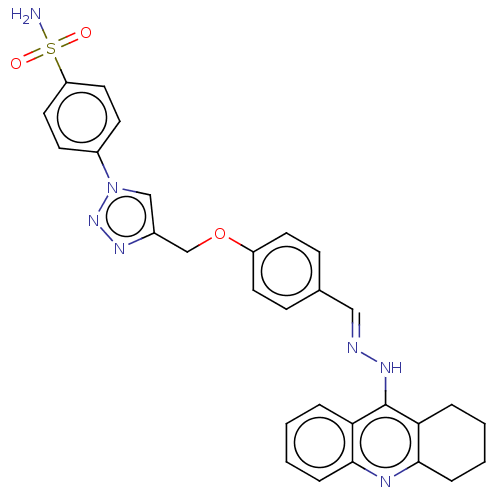 (CHEMBL4444030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -1 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50509706 (CHEMBL4526428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50509704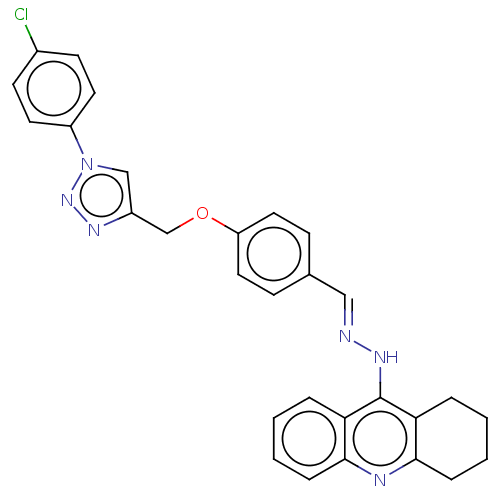 (CHEMBL4443988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509710 (CHEMBL4447258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509716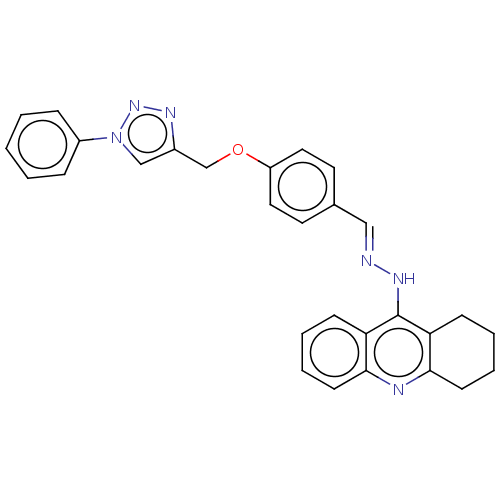 (CHEMBL4445026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50509701 (CHEMBL4592037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509710 (CHEMBL4447258) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509704 (CHEMBL4443988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509707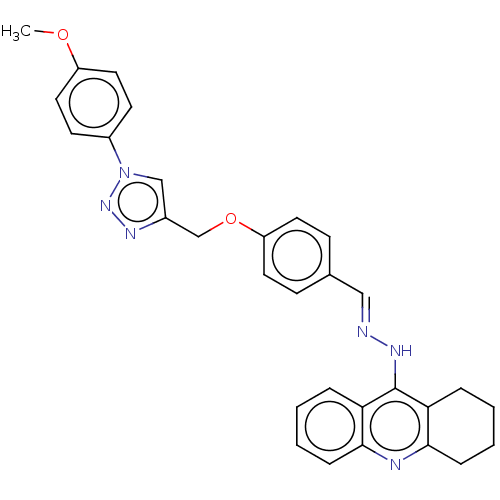 (CHEMBL4553902) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50509707 (CHEMBL4553902) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509701 (CHEMBL4592037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509706 (CHEMBL4526428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509715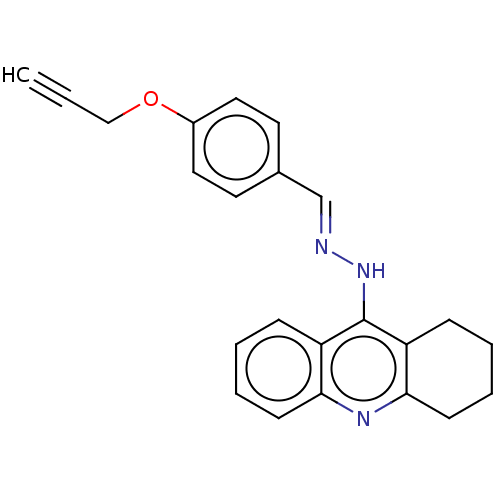 (CHEMBL4542112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50509708 (CHEMBL4458648) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509708 (CHEMBL4458648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509712 (CHEMBL4525409) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509717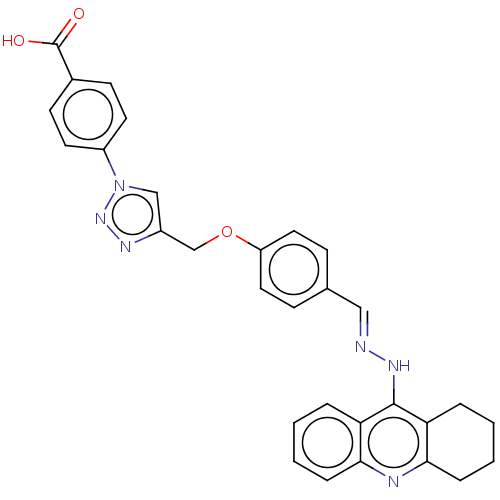 (CHEMBL4557164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509714 (CHEMBL4459154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 307 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509703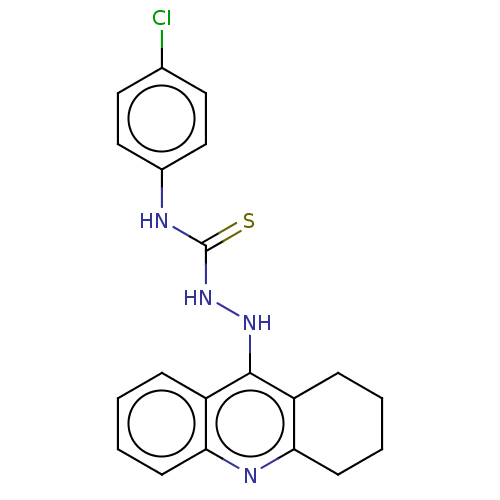 (CHEMBL4435058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509702 (CHEMBL4435178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509715 (CHEMBL4542112) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509709 (CHEMBL4441059) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509718 (CHEMBL4466829) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 547 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509712 (CHEMBL4525409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509702 (CHEMBL4435178) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 571 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509711 (CHEMBL4580216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509708 (CHEMBL4458648) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509713 (CHEMBL4556651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 722 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50509711 (CHEMBL4580216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 748 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509703 (CHEMBL4435058) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 786 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509714 (CHEMBL4459154) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 792 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM13066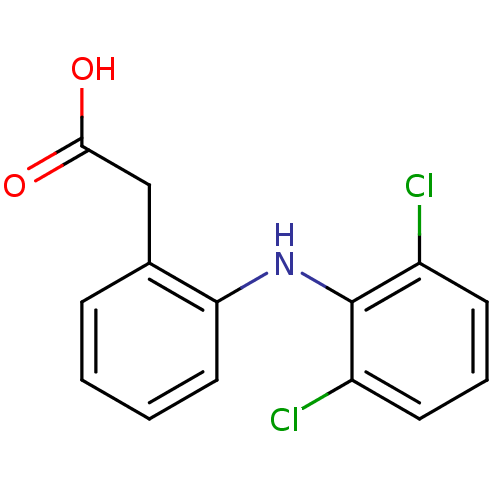 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX -2 by colorimetric inhibitor screening assay kit method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509705 (CHEMBL4444030) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 807 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509702 (CHEMBL4435178) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 861 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509716 (CHEMBL4445026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509712 (CHEMBL4525409) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509716 (CHEMBL4445026) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 917 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509714 (CHEMBL4459154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 961 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509718 (CHEMBL4466829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 BindingDB Entry DOI: 10.7270/Q2V69NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |