 Found 131 hits with Last Name = 'kalinowski' and Initial = 's'
Found 131 hits with Last Name = 'kalinowski' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50585812
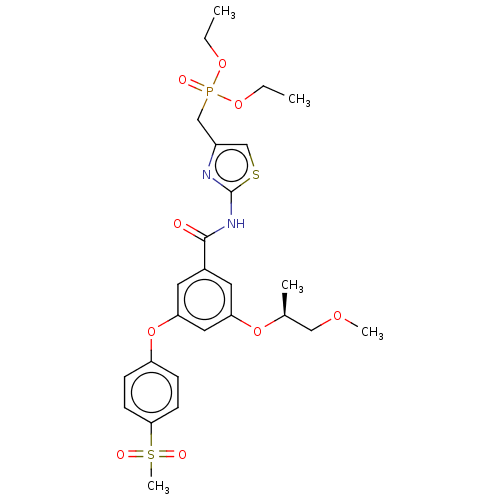
(CHEMBL5091943)Show SMILES CCOP(=O)(Cc1csc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50585813
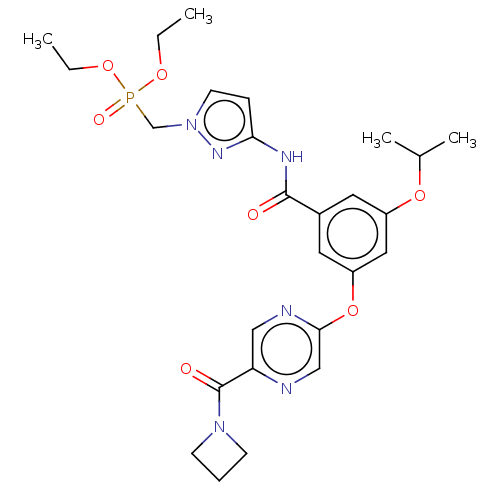
(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581556
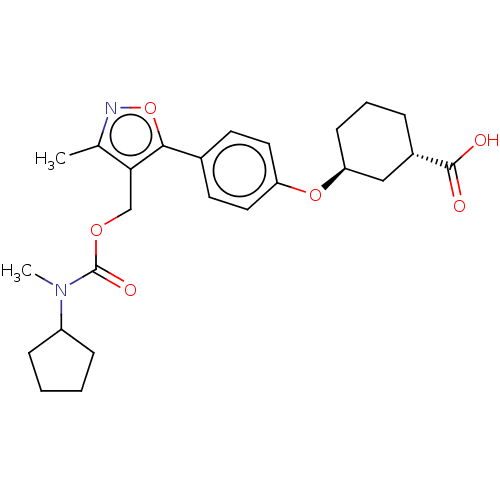
(CHEMBL5085260)Show SMILES CN(C1CCCC1)C(=O)OCc1c(C)noc1-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277463
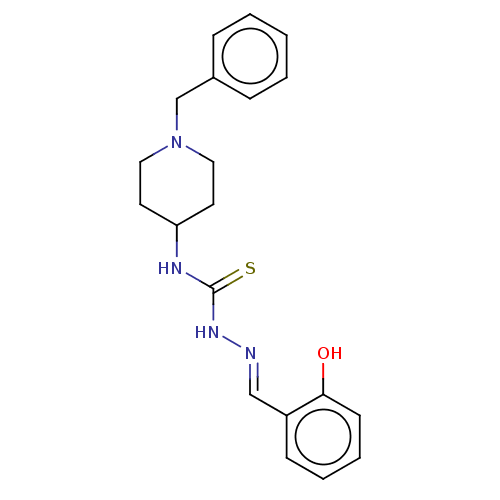
(CHEMBL4163526)Show InChI InChI=1S/C20H24N4OS/c25-19-9-5-4-8-17(19)14-21-23-20(26)22-18-10-12-24(13-11-18)15-16-6-2-1-3-7-16/h1-9,14,18,25H,10-13,15H2,(H2,22,23,26)/b21-14+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277455
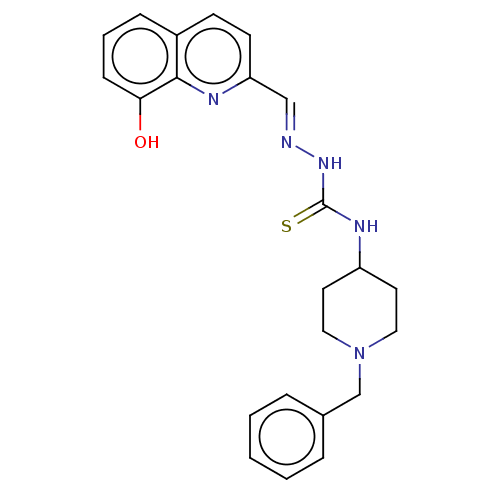
(CHEMBL4175281)Show SMILES Oc1cccc2ccc(\C=N\NC(=S)NC3CCN(Cc4ccccc4)CC3)nc12 Show InChI InChI=1S/C23H25N5OS/c29-21-8-4-7-18-9-10-20(25-22(18)21)15-24-27-23(30)26-19-11-13-28(14-12-19)16-17-5-2-1-3-6-17/h1-10,15,19,29H,11-14,16H2,(H2,26,27,30)/b24-15+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277454
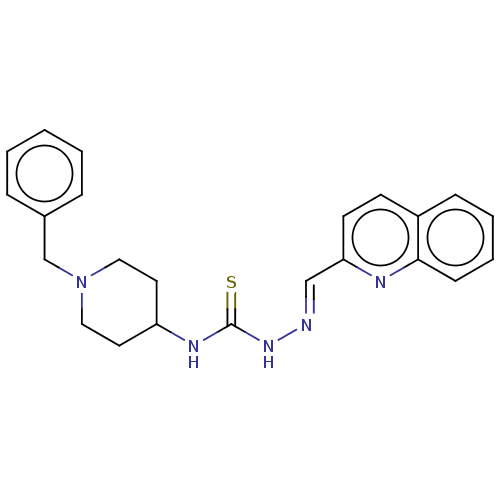
(CHEMBL4168083)Show SMILES S=C(N\N=C\c1ccc2ccccc2n1)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C23H25N5S/c29-23(27-24-16-21-11-10-19-8-4-5-9-22(19)25-21)26-20-12-14-28(15-13-20)17-18-6-2-1-3-7-18/h1-11,16,20H,12-15,17H2,(H2,26,27,29)/b24-16+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277452
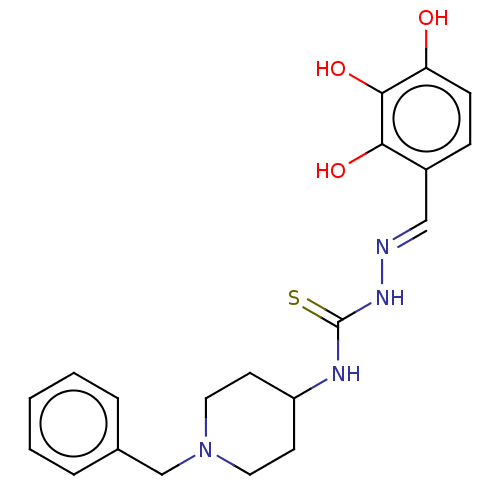
(CHEMBL4162052)Show SMILES Oc1ccc(\C=N\NC(=S)NC2CCN(Cc3ccccc3)CC2)c(O)c1O Show InChI InChI=1S/C20H24N4O3S/c25-17-7-6-15(18(26)19(17)27)12-21-23-20(28)22-16-8-10-24(11-9-16)13-14-4-2-1-3-5-14/h1-7,12,16,25-27H,8-11,13H2,(H2,22,23,28)/b21-12+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277451
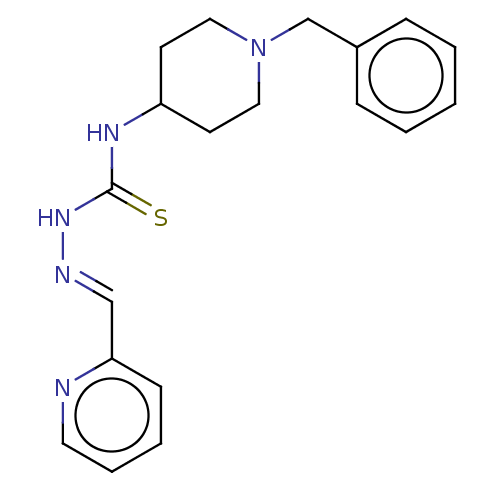
(CHEMBL4160164)Show InChI InChI=1S/C19H23N5S/c25-19(23-21-14-18-8-4-5-11-20-18)22-17-9-12-24(13-10-17)15-16-6-2-1-3-7-16/h1-8,11,14,17H,9-10,12-13,15H2,(H2,22,23,25)/b21-14+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277461

(CHEMBL4164649)Show SMILES Oc1cccc(\C=N\NC(=S)NC2CCN(Cc3ccccc3)CC2)c1O Show InChI InChI=1S/C20H24N4O2S/c25-18-8-4-7-16(19(18)26)13-21-23-20(27)22-17-9-11-24(12-10-17)14-15-5-2-1-3-6-15/h1-8,13,17,25-26H,9-12,14H2,(H2,22,23,27)/b21-13+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277462
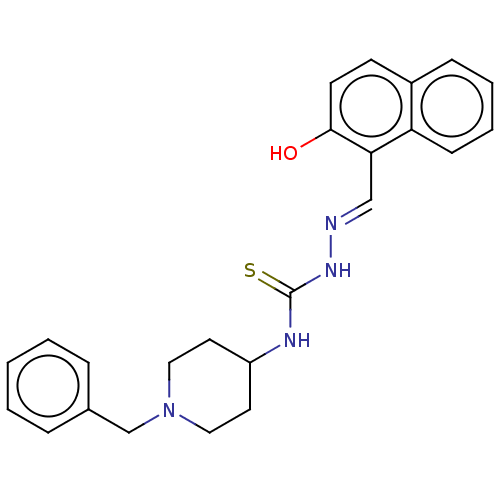
(CHEMBL4171426)Show SMILES Oc1ccc2ccccc2c1\C=N\NC(=S)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C24H26N4OS/c29-23-11-10-19-8-4-5-9-21(19)22(23)16-25-27-24(30)26-20-12-14-28(15-13-20)17-18-6-2-1-3-7-18/h1-11,16,20,29H,12-15,17H2,(H2,26,27,30)/b25-16+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581557
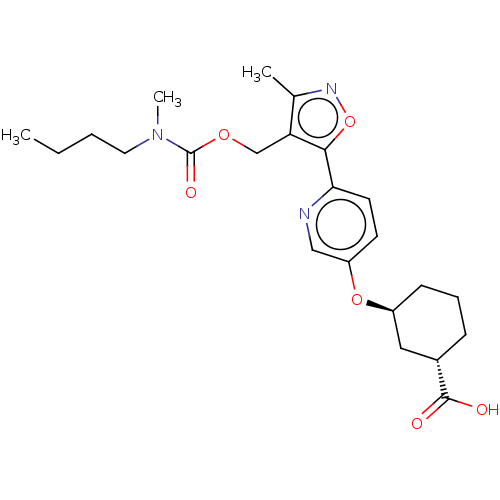
(CHEMBL5070789)Show SMILES CCCCN(C)C(=O)OCc1c(C)noc1-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)cn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581555
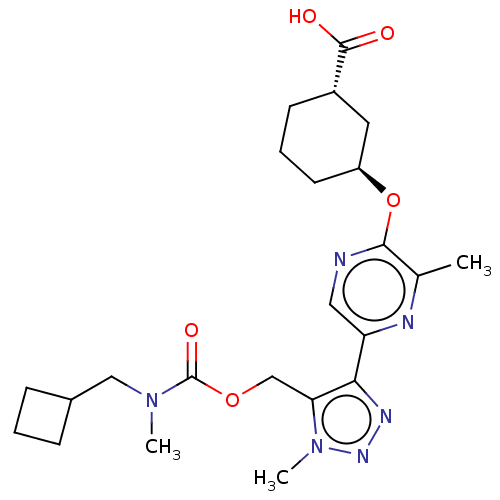
(CHEMBL5085115)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1cnc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BSEP (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50277453
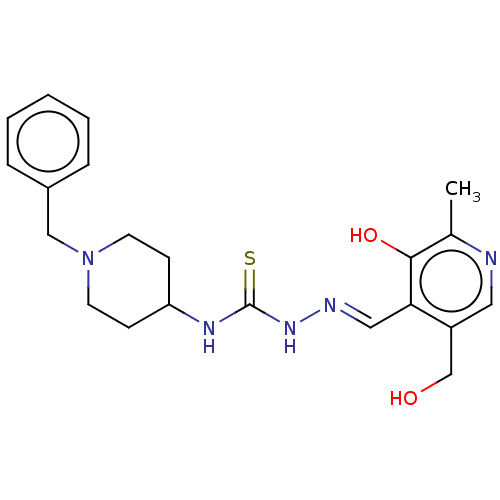
(CHEMBL4174866)Show SMILES Cl.Cc1ncc(CO)c(\C=N\NC(=S)NC2CCN(Cc3ccccc3)CC2)c1O Show InChI InChI=1S/C21H27N5O2S/c1-15-20(28)19(17(14-27)11-22-15)12-23-25-21(29)24-18-7-9-26(10-8-18)13-16-5-3-2-4-6-16/h2-6,11-12,18,27-28H,7-10,13-14H2,1H3,(H2,24,25,29)/b23-12+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured for 5 mi... |
Eur J Med Chem 139: 612-632 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.021
BindingDB Entry DOI: 10.7270/Q2MC92HZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 4
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MRP4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine translocator ABCB4
(Homo sapiens) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDR3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50581555

(CHEMBL5085115)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1cnc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BSEP (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581554
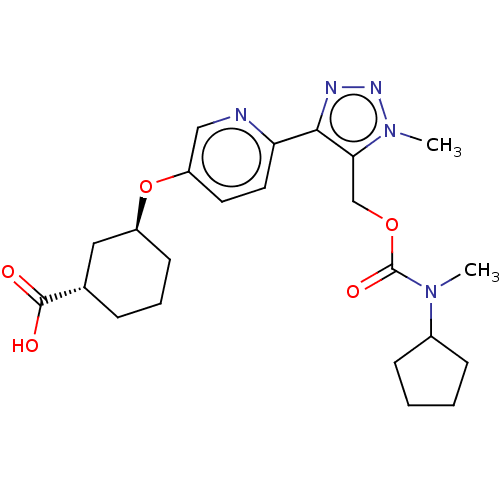
(CHEMBL5092698)Show SMILES CN(C1CCCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)cn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM50581552
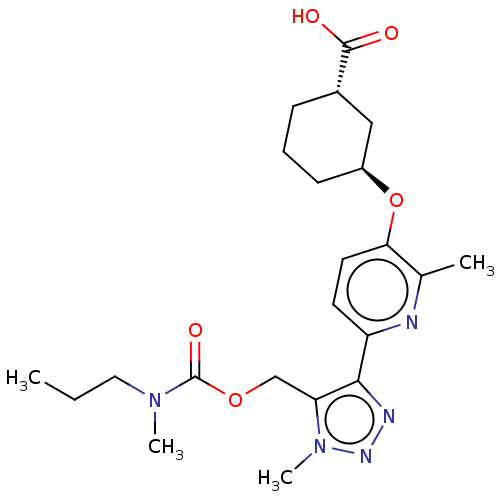
(BMS-986278 | Bms-986278)Show SMILES CCCN(C)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581558
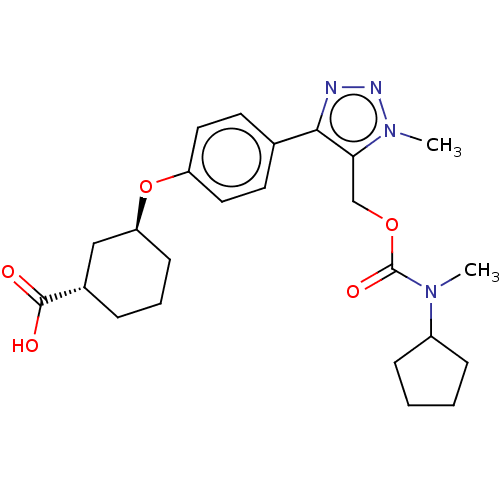
(CHEMBL5083511)Show SMILES CN(C1CCCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 4
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MRP4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BSEP (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 3
(Homo sapiens (Human)) | BDBM50581550

(AP-3152 FREE ACID | BMS-986020 | Bms-986020)Show SMILES C[C@@H](OC(=O)Nc1c(C)noc1-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MRP3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 3
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MRP3 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585829
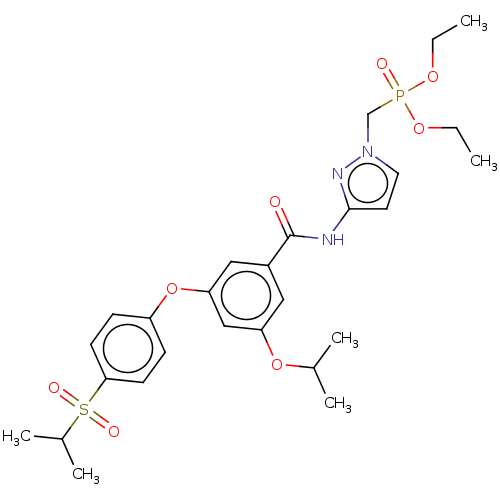
(CHEMBL5092846)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(cc3)S(=O)(=O)C(C)C)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585830
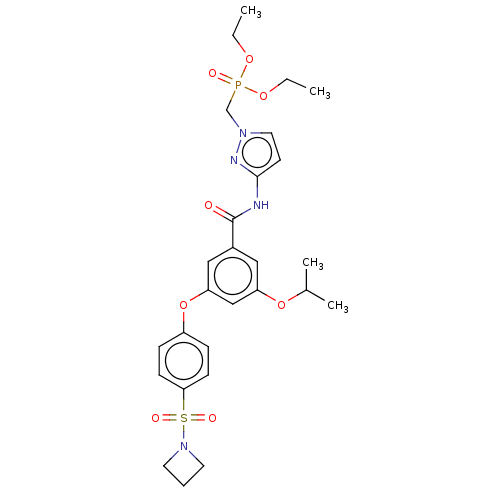
(CHEMBL5072442)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(cc3)S(=O)(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581553
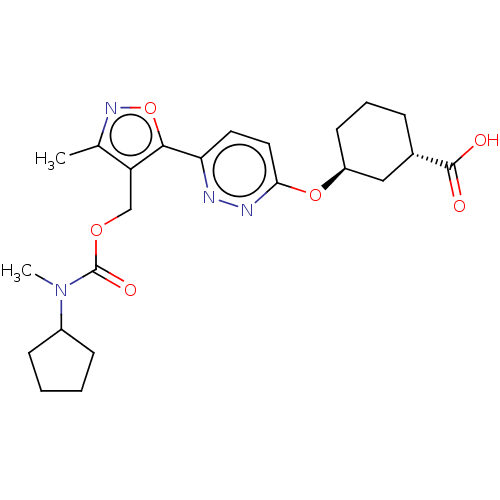
(CHEMBL5070166)Show SMILES CN(C1CCCC1)C(=O)OCc1c(C)noc1-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)nn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50581552

(BMS-986278 | Bms-986278)Show SMILES CCCN(C)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of OATP1B1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50581552

(BMS-986278 | Bms-986278)Show SMILES CCCN(C)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50581552

(BMS-986278 | Bms-986278)Show SMILES CCCN(C)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50581552

(BMS-986278 | Bms-986278)Show SMILES CCCN(C)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50581551

(CHEMBL5072201)Show SMILES CN(CC1CCC1)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50581552

(BMS-986278 | Bms-986278)Show SMILES CCCN(C)C(=O)OCc1c(nnn1C)-c1ccc(O[C@H]2CCC[C@@H](C2)C(O)=O)c(C)n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01256
BindingDB Entry DOI: 10.7270/Q2PZ5DP9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data