 Found 523 hits with Last Name = 'last' and Initial = 's'
Found 523 hits with Last Name = 'last' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250705
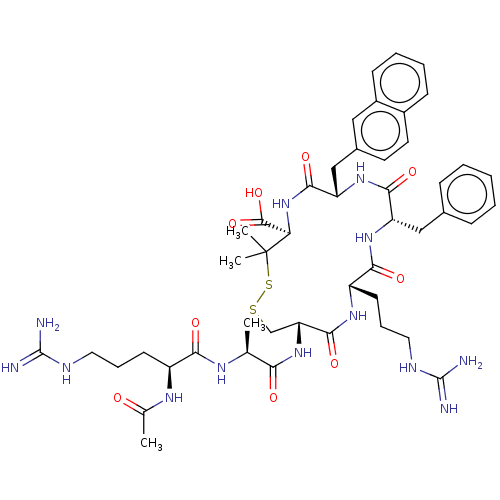
(CHEMBL4078698)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)16-10-20-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)35(24-29-18-19-30-14-8-9-15-31(30)22-29)58-41(65)34(23-28-12-6-5-7-13-28)57-40(64)33(56-43(36)67)17-11-21-53-46(50)51/h5-9,12-15,18-19,22,26,32-37H,10-11,16-17,20-21,23-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,64)(H,58,65)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250733

(CHEMBL4064373)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)16-10-20-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)34(23-28-12-6-5-7-13-28)58-41(65)35(24-29-18-19-30-14-8-9-15-31(30)22-29)57-40(64)33(56-43(36)67)17-11-21-53-46(50)51/h5-9,12-15,18-19,22,26,32-37H,10-11,16-17,20-21,23-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,64)(H,58,65)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250704

(CHEMBL4089168)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C44H63N15O9S2/c1-23(53-36(62)29(54-24(2)60)11-7-15-50-42(45)46)35(61)58-33-21-69-70-44(3,4)34(41(67)68)59-39(65)32(19-28-20-49-22-52-28)57-38(64)31(18-25-13-14-26-9-5-6-10-27(26)17-25)56-37(63)30(55-40(33)66)12-8-16-51-43(47)48/h5-6,9-10,13-14,17,20,22-23,29-34H,7-8,11-12,15-16,18-19,21H2,1-4H3,(H,49,52)(H,53,62)(H,54,60)(H,55,66)(H,56,63)(H,57,64)(H,58,61)(H,59,65)(H,67,68)(H4,45,46,50)(H4,47,48,51)/t23-,29-,30-,31-,32-,33+,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250704

(CHEMBL4089168)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C44H63N15O9S2/c1-23(53-36(62)29(54-24(2)60)11-7-15-50-42(45)46)35(61)58-33-21-69-70-44(3,4)34(41(67)68)59-39(65)32(19-28-20-49-22-52-28)57-38(64)31(18-25-13-14-26-9-5-6-10-27(26)17-25)56-37(63)30(55-40(33)66)12-8-16-51-43(47)48/h5-6,9-10,13-14,17,20,22-23,29-34H,7-8,11-12,15-16,18-19,21H2,1-4H3,(H,49,52)(H,53,62)(H,54,60)(H,55,66)(H,56,63)(H,57,64)(H,58,61)(H,59,65)(H,67,68)(H4,45,46,50)(H4,47,48,51)/t23-,29-,30-,31-,32-,33+,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250710

(CHEMBL4061034)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)19-11-21-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)34(23-28-13-6-5-7-14-28)57-41(65)35(24-30-17-10-16-29-15-8-9-18-31(29)30)58-40(64)33(56-43(36)67)20-12-22-53-46(50)51/h5-10,13-18,26,32-37H,11-12,19-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,65)(H,58,64)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50192468
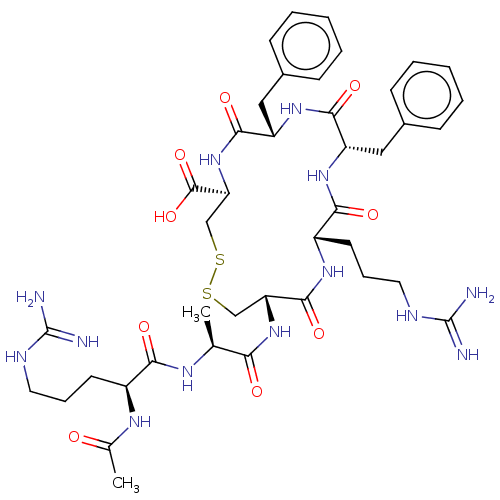
(CHEMBL3923282)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C41H59N13O9S2/c1-23(48-34(57)27(49-24(2)55)15-9-17-46-40(42)43)33(56)53-31-21-64-65-22-32(39(62)63)54-37(60)30(20-26-13-7-4-8-14-26)52-36(59)29(19-25-11-5-3-6-12-25)51-35(58)28(50-38(31)61)16-10-18-47-41(44)45/h3-8,11-14,23,27-32H,9-10,15-22H2,1-2H3,(H,48,57)(H,49,55)(H,50,61)(H,51,58)(H,52,59)(H,53,56)(H,54,60)(H,62,63)(H4,42,43,46)(H4,44,45,47)/t23-,27-,28-,29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50192652
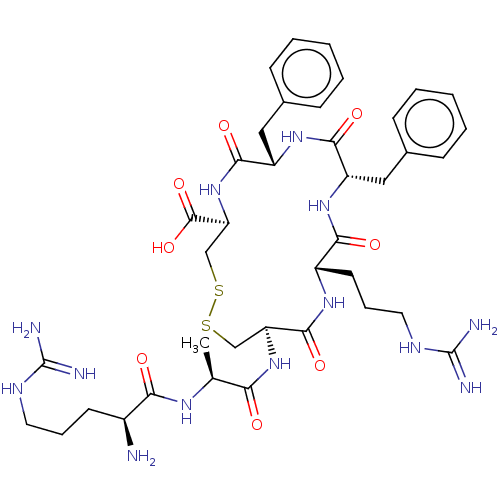
(CHEMBL3901189)Show SMILES C[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C39H57N13O8S2/c1-22(47-32(54)25(40)14-8-16-45-38(41)42)31(53)51-29-20-61-62-21-30(37(59)60)52-35(57)28(19-24-12-6-3-7-13-24)50-34(56)27(18-23-10-4-2-5-11-23)49-33(55)26(48-36(29)58)15-9-17-46-39(43)44/h2-7,10-13,22,25-30H,8-9,14-21,40H2,1H3,(H,47,54)(H,48,58)(H,49,55)(H,50,56)(H,51,53)(H,52,57)(H,59,60)(H4,41,42,45)(H4,43,44,46)/t22-,25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250721

(CHEMBL4093767)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C43H63N13O10S2/c1-23(50-35(60)28(51-24(2)57)12-8-18-48-41(44)45)34(59)55-32-22-67-68-43(3,4)33(40(65)66)56-38(63)31(20-25-10-6-5-7-11-25)54-37(62)30(21-26-14-16-27(58)17-15-26)53-36(61)29(52-39(32)64)13-9-19-49-42(46)47/h5-7,10-11,14-17,23,28-33,58H,8-9,12-13,18-22H2,1-4H3,(H,50,60)(H,51,57)(H,52,64)(H,53,61)(H,54,62)(H,55,59)(H,56,63)(H,65,66)(H4,44,45,48)(H4,46,47,49)/t23-,28-,29-,30-,31-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250732
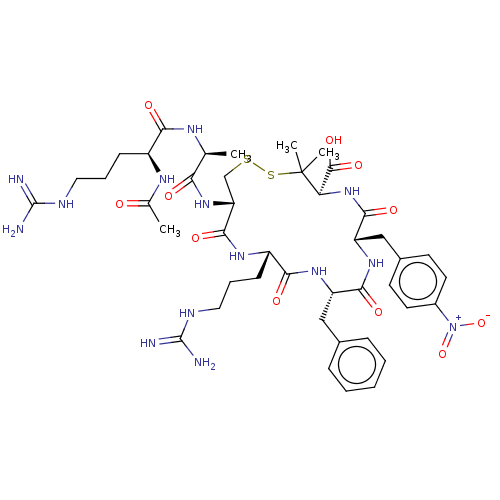
(CHEMBL4064578)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C43H62N14O11S2/c1-23(50-35(60)28(51-24(2)58)12-8-18-48-41(44)45)34(59)55-32-22-69-70-43(3,4)33(40(65)66)56-38(63)31(21-26-14-16-27(17-15-26)57(67)68)54-37(62)30(20-25-10-6-5-7-11-25)53-36(61)29(52-39(32)64)13-9-19-49-42(46)47/h5-7,10-11,14-17,23,28-33H,8-9,12-13,18-22H2,1-4H3,(H,50,60)(H,51,58)(H,52,64)(H,53,61)(H,54,62)(H,55,59)(H,56,63)(H,65,66)(H4,44,45,48)(H4,46,47,49)/t23-,28-,29-,30-,31-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249644

(CHEMBL4073895)Show SMILES Nc1nc(NCc2cc(on2)C2CC2)c2n(Cc3ccccn3)ccc2n1 Show InChI InChI=1S/C19H19N7O/c20-19-23-15-6-8-26(11-13-3-1-2-7-21-13)17(15)18(24-19)22-10-14-9-16(27-25-14)12-4-5-12/h1-3,6-9,12H,4-5,10-11H2,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250711
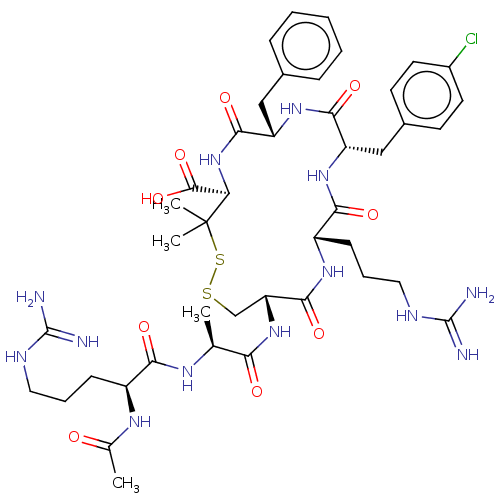
(CHEMBL4065967)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C43H62ClN13O9S2/c1-23(51-35(60)28(52-24(2)58)12-8-18-49-41(45)46)34(59)56-32-22-67-68-43(3,4)33(40(65)66)57-38(63)31(20-25-10-6-5-7-11-25)55-37(62)30(21-26-14-16-27(44)17-15-26)54-36(61)29(53-39(32)64)13-9-19-50-42(47)48/h5-7,10-11,14-17,23,28-33H,8-9,12-13,18-22H2,1-4H3,(H,51,60)(H,52,58)(H,53,64)(H,54,61)(H,55,62)(H,56,59)(H,57,63)(H,65,66)(H4,45,46,49)(H4,47,48,50)/t23-,28-,29-,30-,31-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50454647
(CHEMBL4209700)Show InChI InChI=1S/C19H22N4O/c1-2-3-12-21-18-17-15(22-19(20)23-18)10-7-11-16(17)24-13-14-8-5-4-6-9-14/h4-11H,2-3,12-13H2,1H3,(H3,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50192468

(CHEMBL3923282)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C41H59N13O9S2/c1-23(48-34(57)27(49-24(2)55)15-9-17-46-40(42)43)33(56)53-31-21-64-65-22-32(39(62)63)54-37(60)30(20-26-13-7-4-8-14-26)52-36(59)29(19-25-11-5-3-6-12-25)51-35(58)28(50-38(31)61)16-10-18-47-41(44)45/h3-8,11-14,23,27-32H,9-10,15-22H2,1-2H3,(H,48,57)(H,49,55)(H,50,61)(H,51,58)(H,52,59)(H,53,56)(H,54,60)(H,62,63)(H4,42,43,46)(H4,44,45,47)/t23-,27-,28-,29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250709
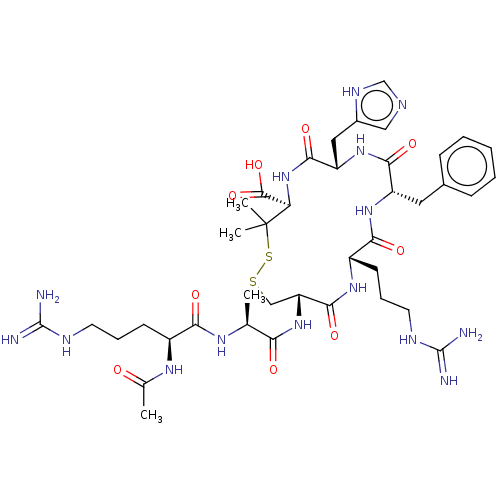
(CHEMBL4076358)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C40H61N15O9S2/c1-21(49-32(58)25(50-22(2)56)12-8-14-46-38(41)42)31(57)54-29-19-65-66-40(3,4)30(37(63)64)55-35(61)28(17-24-18-45-20-48-24)53-34(60)27(16-23-10-6-5-7-11-23)52-33(59)26(51-36(29)62)13-9-15-47-39(43)44/h5-7,10-11,18,20-21,25-30H,8-9,12-17,19H2,1-4H3,(H,45,48)(H,49,58)(H,50,56)(H,51,62)(H,52,59)(H,53,60)(H,54,57)(H,55,61)(H,63,64)(H4,41,42,46)(H4,43,44,47)/t21-,25-,26-,27-,28-,29+,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249651
(CHEMBL4098533)Show SMILES Cc1cc(CNc2nc(N)nc3ccn(Cc4cc(Cl)ccn4)c23)no1 Show InChI InChI=1S/C17H16ClN7O/c1-10-6-12(24-26-10)8-21-16-15-14(22-17(19)23-16)3-5-25(15)9-13-7-11(18)2-4-20-13/h2-7H,8-9H2,1H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249650
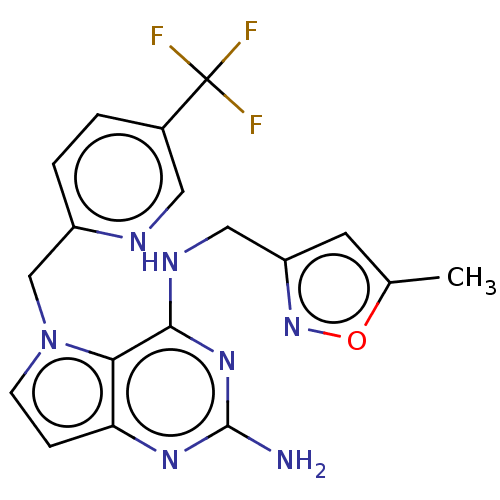
(CHEMBL4088372)Show SMILES Cc1cc(CNc2nc(N)nc3ccn(Cc4ccc(cn4)C(F)(F)F)c23)no1 Show InChI InChI=1S/C18H16F3N7O/c1-10-6-13(27-29-10)8-24-16-15-14(25-17(22)26-16)4-5-28(15)9-12-3-2-11(7-23-12)18(19,20)21/h2-7H,8-9H2,1H3,(H3,22,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281369

(CHEMBL4162750)Show InChI InChI=1S/C16H24N4O/c1-3-4-7-12(9-10-21)18-15-14-11(2)6-5-8-13(14)19-16(17)20-15/h5-6,8,12,21H,3-4,7,9-10H2,1-2H3,(H3,17,18,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50281371

(CHEMBL4171368)Show InChI InChI=1S/C15H22N4O2/c1-3-4-10(7-8-20)17-14-12-6-5-11(21-2)9-13(12)18-15(16)19-14/h5-6,9-10,20H,3-4,7-8H2,1-2H3,(H3,16,17,18,19)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50249723
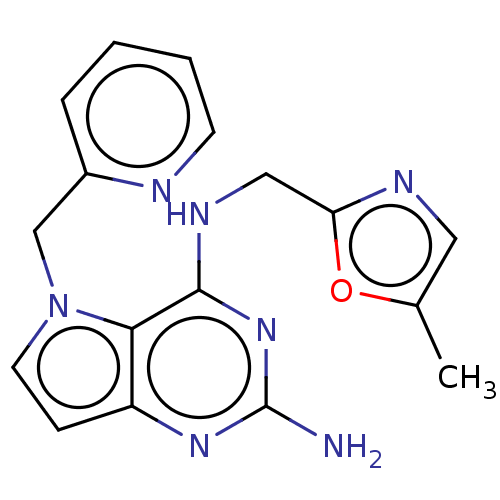
(CHEMBL4070546)Show InChI InChI=1S/C17H17N7O/c1-11-8-20-14(25-11)9-21-16-15-13(22-17(18)23-16)5-7-24(15)10-12-4-2-3-6-19-12/h2-8H,9-10H2,1H3,(H3,18,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor (unknown origin) |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249645

(CHEMBL4078859)Show SMILES COc1ccc(Cn2ccc3nc(N)nc(NCc4cc(C)on4)c23)nc1OC Show InChI InChI=1S/C19H21N7O3/c1-11-8-13(25-29-11)9-21-17-16-14(23-19(20)24-17)6-7-26(16)10-12-4-5-15(27-2)18(22-12)28-3/h4-8H,9-10H2,1-3H3,(H3,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50454641
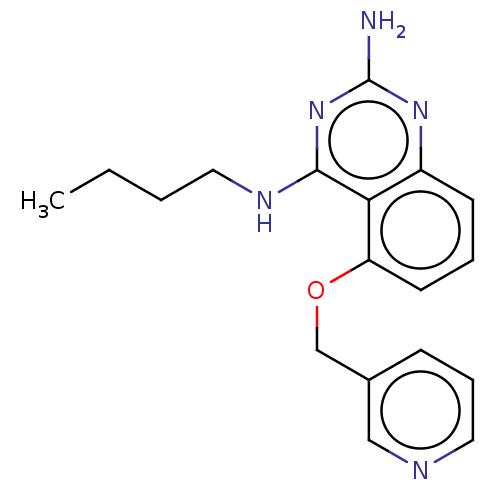
(CHEMBL4206363)Show InChI InChI=1S/C18H21N5O/c1-2-3-10-21-17-16-14(22-18(19)23-17)7-4-8-15(16)24-12-13-6-5-9-20-11-13/h4-9,11H,2-3,10,12H2,1H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249731

(CHEMBL4080513)Show InChI InChI=1S/C17H16ClN7O/c1-10-7-11(24-26-10)8-21-16-15-13(22-17(19)23-16)4-6-25(15)9-14-12(18)3-2-5-20-14/h2-7H,8-9H2,1H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249699
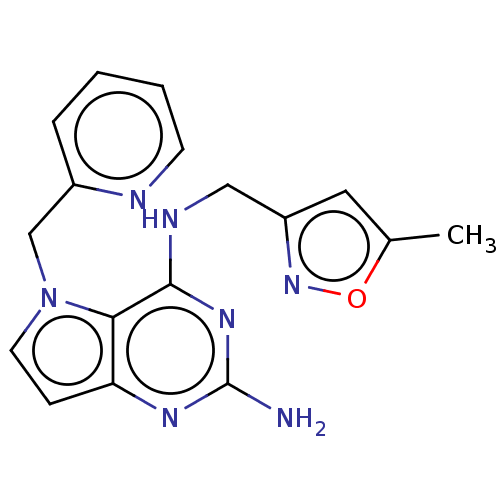
(CHEMBL4094458)Show InChI InChI=1S/C17H17N7O/c1-11-8-13(23-25-11)9-20-16-15-14(21-17(18)22-16)5-7-24(15)10-12-4-2-3-6-19-12/h2-8H,9-10H2,1H3,(H3,18,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250708

(CHEMBL4079246)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C43H63N13O10S2/c1-23(50-35(60)28(51-24(2)57)12-8-18-48-41(44)45)34(59)55-32-22-67-68-43(3,4)33(40(65)66)56-38(63)31(21-26-14-16-27(58)17-15-26)54-37(62)30(20-25-10-6-5-7-11-25)53-36(61)29(52-39(32)64)13-9-19-49-42(46)47/h5-7,10-11,14-17,23,28-33,58H,8-9,12-13,18-22H2,1-4H3,(H,50,60)(H,51,57)(H,52,64)(H,53,61)(H,54,62)(H,55,59)(H,56,63)(H,65,66)(H4,44,45,48)(H4,46,47,49)/t23-,28-,29-,30-,31-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50454640
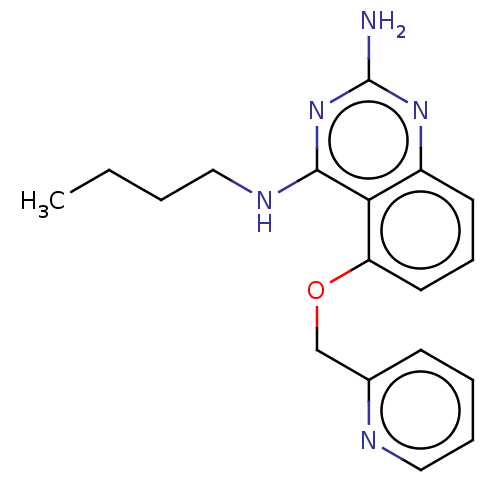
(CHEMBL4218074)Show InChI InChI=1S/C18H21N5O/c1-2-3-10-21-17-16-14(22-18(19)23-17)8-6-9-15(16)24-12-13-7-4-5-11-20-13/h4-9,11H,2-3,10,12H2,1H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250723
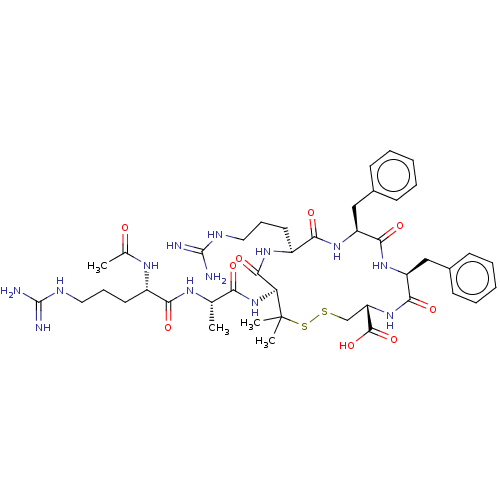
(CHEMBL4060078)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CSSC1(C)C)C(O)=O |r| Show InChI InChI=1S/C43H63N13O9S2/c1-24(50-35(59)28(51-25(2)57)17-11-19-48-41(44)45)34(58)56-33-39(63)52-29(18-12-20-49-42(46)47)36(60)53-30(21-26-13-7-5-8-14-26)37(61)54-31(22-27-15-9-6-10-16-27)38(62)55-32(40(64)65)23-66-67-43(33,3)4/h5-10,13-16,24,28-33H,11-12,17-23H2,1-4H3,(H,50,59)(H,51,57)(H,52,63)(H,53,60)(H,54,61)(H,55,62)(H,56,58)(H,64,65)(H4,44,45,48)(H4,46,47,49)/t24-,28-,29-,30-,31-,32-,33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249624

(CHEMBL4095178)Show InChI InChI=1S/C18H19N7O2/c1-11-8-12(24-27-11)9-21-17-16-13(22-18(19)23-17)5-7-25(16)10-14-15(26-2)4-3-6-20-14/h3-8H,9-10H2,1-2H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50281371

(CHEMBL4171368)Show InChI InChI=1S/C15H22N4O2/c1-3-4-10(7-8-20)17-14-12-6-5-11(21-2)9-13(12)18-15(16)19-14/h5-6,9-10,20H,3-4,7-8H2,1-2H3,(H3,16,17,18,19)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor (unknown origin) |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250706
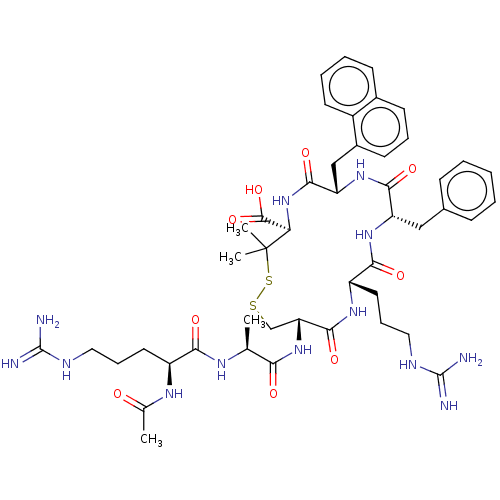
(CHEMBL4096721)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)19-11-21-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)35(24-30-17-10-16-29-15-8-9-18-31(29)30)58-41(65)34(23-28-13-6-5-7-14-28)57-40(64)33(56-43(36)67)20-12-22-53-46(50)51/h5-10,13-18,26,32-37H,11-12,19-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,64)(H,58,65)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281374

(CHEMBL4160103)Show InChI InChI=1S/C16H24N4O/c1-3-4-5-12(8-9-21)18-15-13-7-6-11(2)10-14(13)19-16(17)20-15/h6-7,10,12,21H,3-5,8-9H2,1-2H3,(H3,17,18,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249728
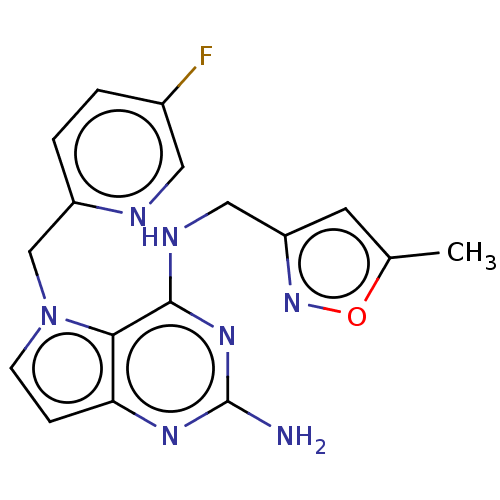
(CHEMBL4072841)Show InChI InChI=1S/C17H16FN7O/c1-10-6-13(24-26-10)8-21-16-15-14(22-17(19)23-16)4-5-25(15)9-12-3-2-11(18)7-20-12/h2-7H,8-9H2,1H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249647

(CHEMBL4099725)Show InChI InChI=1S/C17H16FN7O/c1-10-7-11(24-26-10)8-21-16-15-13(22-17(19)23-16)4-6-25(15)9-14-12(18)3-2-5-20-14/h2-7H,8-9H2,1H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250725
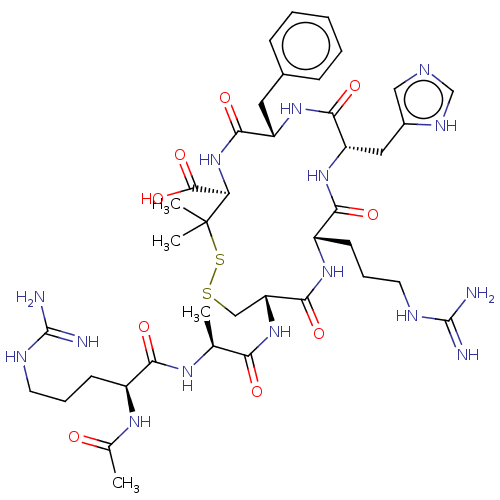
(CHEMBL4073066)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C40H61N15O9S2/c1-21(49-32(58)25(50-22(2)56)12-8-14-46-38(41)42)31(57)54-29-19-65-66-40(3,4)30(37(63)64)55-35(61)27(16-23-10-6-5-7-11-23)52-34(60)28(17-24-18-45-20-48-24)53-33(59)26(51-36(29)62)13-9-15-47-39(43)44/h5-7,10-11,18,20-21,25-30H,8-9,12-17,19H2,1-4H3,(H,45,48)(H,49,58)(H,50,56)(H,51,62)(H,52,60)(H,53,59)(H,54,57)(H,55,61)(H,63,64)(H4,41,42,46)(H4,43,44,47)/t21-,25-,26-,27-,28-,29+,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249623

(CHEMBL4074704)Show InChI InChI=1S/C18H18N6O3/c1-11-8-21-15(27-11)10-26-17-16-12(22-18(19)23-17)5-7-24(16)9-13-14(25-2)4-3-6-20-13/h3-8H,9-10H2,1-2H3,(H2,19,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249646
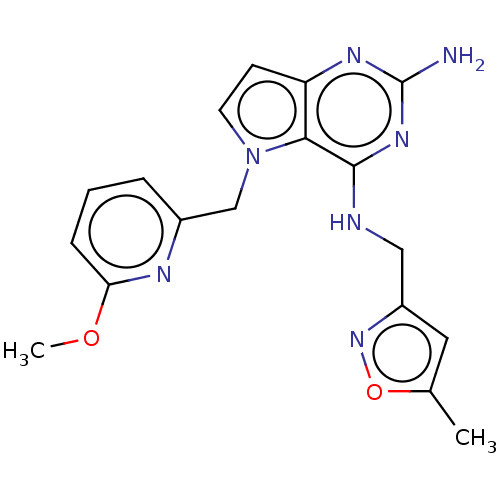
(CHEMBL4103135)Show SMILES COc1cccc(Cn2ccc3nc(N)nc(NCc4cc(C)on4)c23)n1 Show InChI InChI=1S/C18H19N7O2/c1-11-8-13(24-27-11)9-20-17-16-14(22-18(19)23-17)6-7-25(16)10-12-4-3-5-15(21-12)26-2/h3-8H,9-10H2,1-2H3,(H3,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Displacement of 3H-dofetilide from human ERG |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50249664

(CHEMBL4065786)Show InChI InChI=1S/C17H17N7O/c1-11-10-25-14(21-11)8-20-16-15-13(22-17(18)23-16)5-7-24(15)9-12-4-2-3-6-19-12/h2-7,10H,8-9H2,1H3,(H3,18,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica , N. V. Turnhoutseweg 30, 2340 Beerse, Belgium.
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor (unknown origin) |
J Med Chem 60: 6137-6151 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00365
BindingDB Entry DOI: 10.7270/Q2XP77CC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50281371

(CHEMBL4171368)Show InChI InChI=1S/C15H22N4O2/c1-3-4-10(7-8-20)17-14-12-6-5-11(21-2)9-13(12)18-15(16)19-14/h5-6,9-10,20H,3-4,7-8H2,1-2H3,(H3,16,17,18,19)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
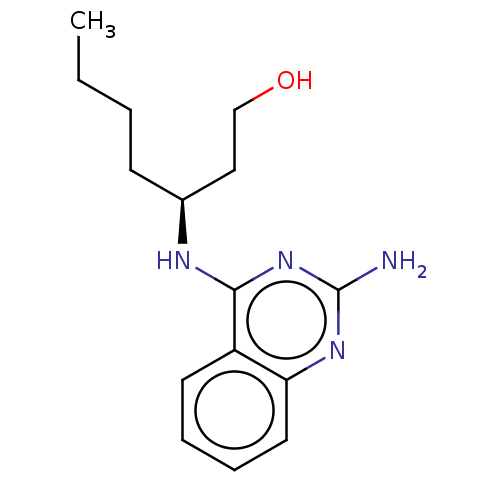
(Homo sapiens (Human)) | BDBM50281377
(CHEMBL4170169)Show InChI InChI=1S/C15H22N4O/c1-2-3-6-11(9-10-20)17-14-12-7-4-5-8-13(12)18-15(16)19-14/h4-5,7-8,11,20H,2-3,6,9-10H2,1H3,(H3,16,17,18,19)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
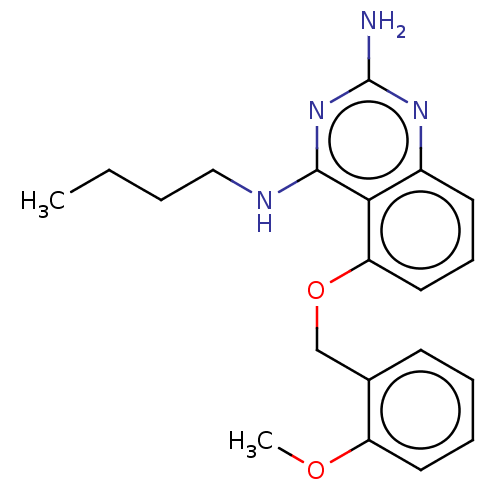
(Homo sapiens (Human)) | BDBM50454639
(CHEMBL4208231)Show InChI InChI=1S/C20H24N4O2/c1-3-4-12-22-19-18-15(23-20(21)24-19)9-7-11-17(18)26-13-14-8-5-6-10-16(14)25-2/h5-11H,3-4,12-13H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281375

(CHEMBL4166919)Show InChI InChI=1S/C16H24N4O2/c1-3-4-5-11(8-9-21)18-15-13-7-6-12(22-2)10-14(13)19-16(17)20-15/h6-7,10-11,21H,3-5,8-9H2,1-2H3,(H3,17,18,19,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250724
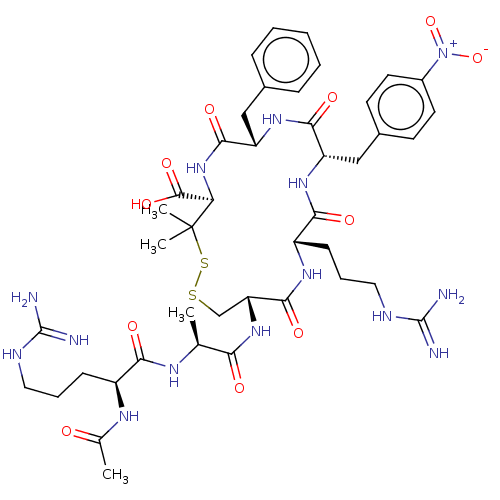
(CHEMBL4074277)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C43H62N14O11S2/c1-23(50-35(60)28(51-24(2)58)12-8-18-48-41(44)45)34(59)55-32-22-69-70-43(3,4)33(40(65)66)56-38(63)31(20-25-10-6-5-7-11-25)54-37(62)30(21-26-14-16-27(17-15-26)57(67)68)53-36(61)29(52-39(32)64)13-9-19-49-42(46)47/h5-7,10-11,14-17,23,28-33H,8-9,12-13,18-22H2,1-4H3,(H,50,60)(H,51,58)(H,52,64)(H,53,61)(H,54,62)(H,55,59)(H,56,63)(H,65,66)(H4,44,45,48)(H4,46,47,49)/t23-,28-,29-,30-,31-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50454646

(CHEMBL4205861)Show InChI InChI=1S/C20H25N5O3/c1-4-5-10-23-19-17-13(24-20(21)25-19)7-6-8-15(17)28-12-14-18(27-3)16(26-2)9-11-22-14/h6-9,11H,4-5,10,12H2,1-3H3,(H3,21,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50454637
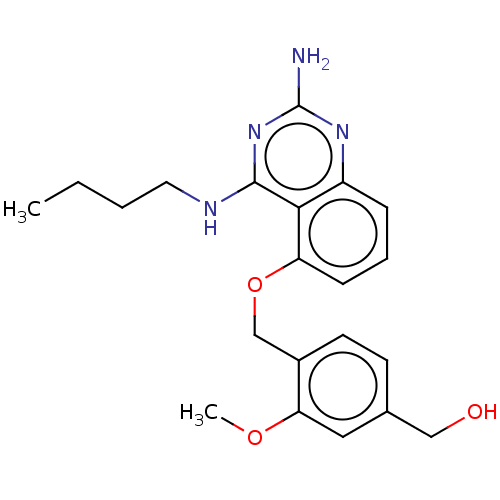
(CHEMBL4213155)Show InChI InChI=1S/C21H26N4O3/c1-3-4-10-23-20-19-16(24-21(22)25-20)6-5-7-17(19)28-13-15-9-8-14(12-26)11-18(15)27-2/h5-9,11,26H,3-4,10,12-13H2,1-2H3,(H3,22,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281384

(CHEMBL4170320)Show InChI InChI=1S/C15H21FN4O/c1-2-3-5-10(8-9-21)18-14-11-6-4-7-12(16)13(11)19-15(17)20-14/h4,6-7,10,21H,2-3,5,8-9H2,1H3,(H3,17,18,19,20)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281383
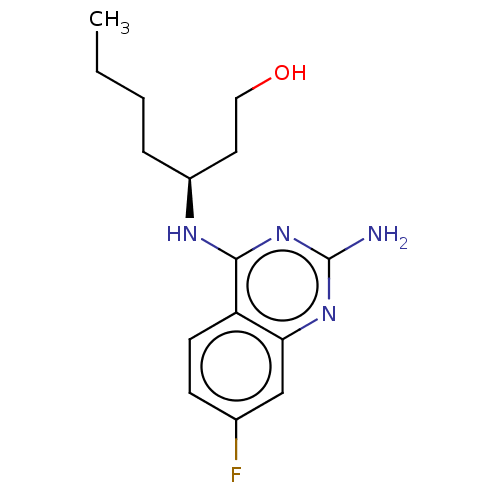
(CHEMBL4163469)Show InChI InChI=1S/C15H21FN4O/c1-2-3-4-11(7-8-21)18-14-12-6-5-10(16)9-13(12)19-15(17)20-14/h5-6,9,11,21H,2-4,7-8H2,1H3,(H3,17,18,19,20)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50454637

(CHEMBL4213155)Show InChI InChI=1S/C21H26N4O3/c1-3-4-10-23-20-19-16(24-21(22)25-20)6-5-7-17(19)28-13-15-9-8-14(12-26)11-18(15)27-2/h5-9,11,26H,3-4,10,12-13H2,1-2H3,(H3,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281380
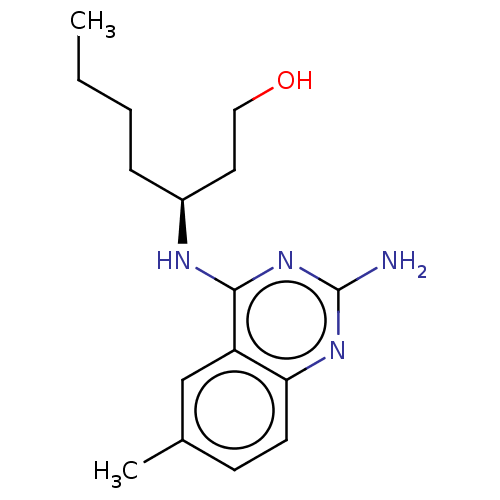
(CHEMBL4174506)Show InChI InChI=1S/C16H24N4O/c1-3-4-5-12(8-9-21)18-15-13-10-11(2)6-7-14(13)19-16(17)20-15/h6-7,10,12,21H,3-5,8-9H2,1-2H3,(H3,17,18,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50281378

(CHEMBL4175502)Show InChI InChI=1S/C15H22N4O/c1-3-5-11(8-9-20)17-14-13-10(2)6-4-7-12(13)18-15(16)19-14/h4,6-7,11,20H,3,5,8-9H2,1-2H3,(H3,16,17,18,19)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases-Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human ERG expressed in HEK293 cell membranes by scintillation counting method |
J Med Chem 61: 6236-6246 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00643
BindingDB Entry DOI: 10.7270/Q2057JFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50454643
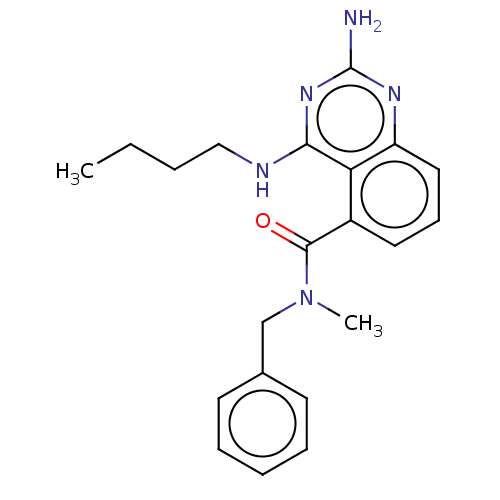
(CHEMBL4218623)Show InChI InChI=1S/C21H25N5O/c1-3-4-13-23-19-18-16(11-8-12-17(18)24-21(22)25-19)20(27)26(2)14-15-9-6-5-7-10-15/h5-12H,3-4,13-14H2,1-2H3,(H3,22,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50454647

(CHEMBL4209700)Show InChI InChI=1S/C19H22N4O/c1-2-3-12-21-18-17-15(22-19(20)23-18)10-7-11-16(17)24-13-14-8-5-4-6-9-14/h4-11H,2-3,12-13H2,1H3,(H3,20,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Infectious Diseases Diagnostics BVBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 28: 711-719 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.014
BindingDB Entry DOI: 10.7270/Q2K35X8K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data