

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50403106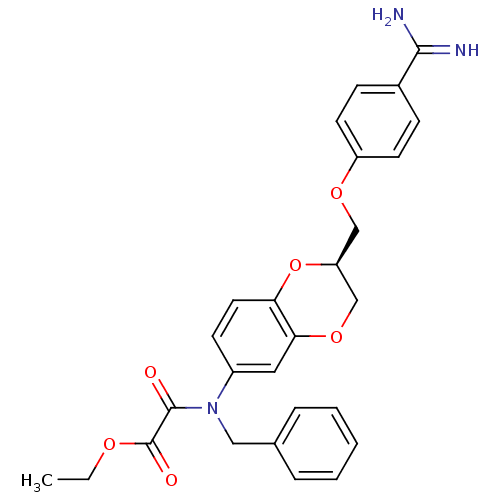 (CHEMBL2216916) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of thrombin | Eur J Med Chem 58: 160-70 (2012) Article DOI: 10.1016/j.ejmech.2012.10.001 BindingDB Entry DOI: 10.7270/Q2J38TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50403105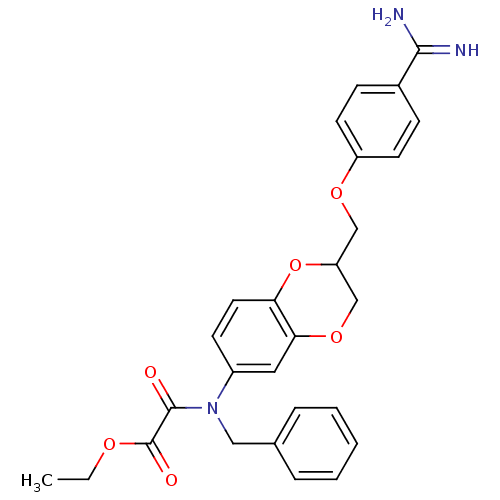 (CHEMBL2216917) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of thrombin | Eur J Med Chem 58: 160-70 (2012) Article DOI: 10.1016/j.ejmech.2012.10.001 BindingDB Entry DOI: 10.7270/Q2J38TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 h... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 15 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 15... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340677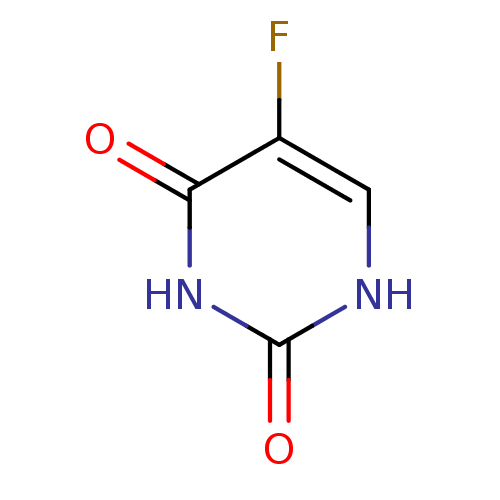 ((5-fluorouracil)5-Fluoro-1H-pyrimidine-2,4-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340677 ((5-fluorouracil)5-Fluoro-1H-pyrimidine-2,4-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340675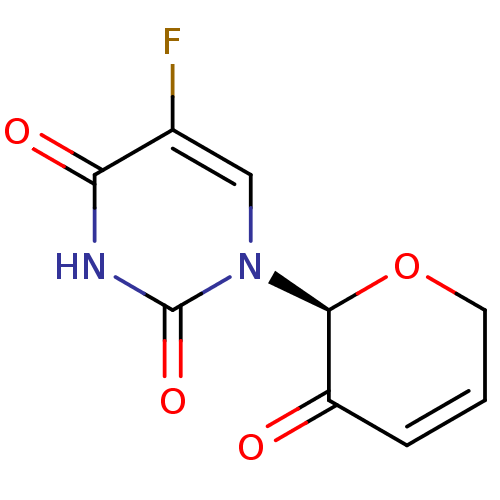 ((S)-5-fluoro-4-hydroxy-1-(3-oxo-3,6-dihydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340675 ((S)-5-fluoro-4-hydroxy-1-(3-oxo-3,6-dihydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340677 ((5-fluorouracil)5-Fluoro-1H-pyrimidine-2,4-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 h... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340676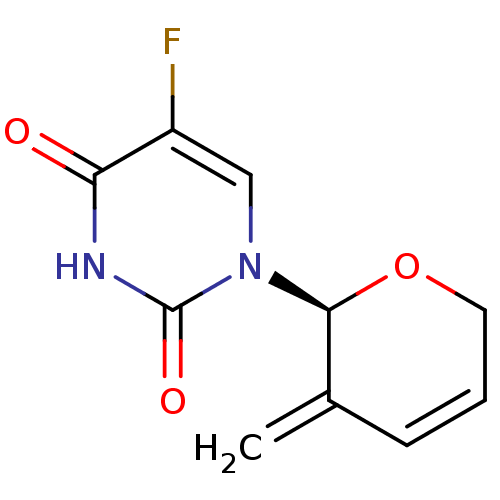 (CHEMBL1762225 | exo-(S)-5-fluoro-4-hydroxy-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340679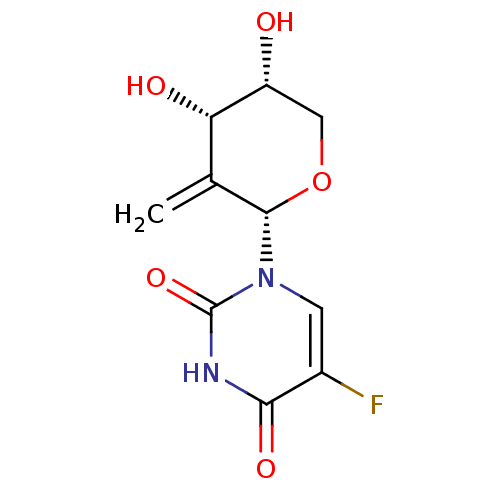 (CHEMBL1762230 | exo-1-((2S,4S,5R)-4,5-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 24 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340677 ((5-fluorouracil)5-Fluoro-1H-pyrimidine-2,4-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340675 ((S)-5-fluoro-4-hydroxy-1-(3-oxo-3,6-dihydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 h... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340675 ((S)-5-fluoro-4-hydroxy-1-(3-oxo-3,6-dihydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340676 (CHEMBL1762225 | exo-(S)-5-fluoro-4-hydroxy-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194179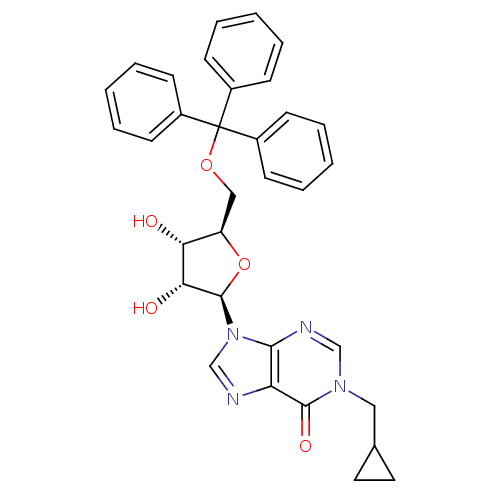 (1-(cyclopropyl)methyl-5'-O-tritylinosine | CHEMBL3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340679 (CHEMBL1762230 | exo-1-((2S,4S,5R)-4,5-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 h... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194181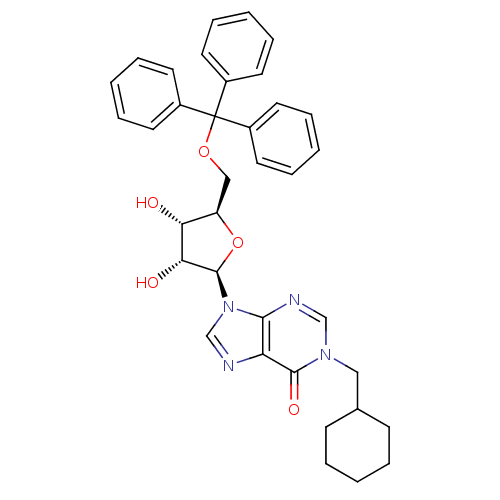 (1-(cyclohexyl)methyl-5'-O-tritylinosine | CHEMBL21...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194173 (5'-O-[(4-chlorophenyl)-1,1-(diphenyl)methyl]inosin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340679 (CHEMBL1762230 | exo-1-((2S,4S,5R)-4,5-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 24... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340679 (CHEMBL1762230 | exo-1-((2S,4S,5R)-4,5-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340677 ((5-fluorouracil)5-Fluoro-1H-pyrimidine-2,4-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 15 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340675 ((S)-5-fluoro-4-hydroxy-1-(3-oxo-3,6-dihydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 15 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340679 (CHEMBL1762230 | exo-1-((2S,4S,5R)-4,5-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 15... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340676 (CHEMBL1762225 | exo-(S)-5-fluoro-4-hydroxy-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 4 h... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340676 (CHEMBL1762225 | exo-(S)-5-fluoro-4-hydroxy-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 4 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340676 (CHEMBL1762225 | exo-(S)-5-fluoro-4-hydroxy-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxyuridine after preincubation for 15 ... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340676 (CHEMBL1762225 | exo-(S)-5-fluoro-4-hydroxy-1-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 15... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340675 ((S)-5-fluoro-4-hydroxy-1-(3-oxo-3,6-dihydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase in mouse L1210 cells assessed as inhibition of tritium release from [5-3H]deoxycytidine after preincubation for 15... | Eur J Med Chem 46: 993-1005 (2011) Article DOI: 10.1016/j.ejmech.2011.01.005 BindingDB Entry DOI: 10.7270/Q2X34XS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194174 (1-benzyl-5'-O-tritylinosine | CHEMBL441540) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194175 (1-allyl-5'-O-tritylinosine | CHEMBL213891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194170 (1-propyl-5'-O-tritylinosine | CHEMBL213890) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194176 (5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194168 (9-((2R,3R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194184 (5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194186 (2',3'-Di-O-acetyl-5'-O-trityl-1-[(2-methoxyethoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50365128 (CHEMBL1949835 | CHEMBL2216906) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR2 expressed in Sf9 insect cells assessed as inhibition of poly(Glu,Tyr) 4:1 substrate phosphorylation by radiome... | Eur J Med Chem 58: 160-70 (2012) Article DOI: 10.1016/j.ejmech.2012.10.001 BindingDB Entry DOI: 10.7270/Q2J38TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194185 (5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194180 (1-[(E)-3-methoxycarbonyl-2-propenyl]-5'-O-tritylin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194178 (6-chloro-9-(5-O-trityl-beta-D-ribofuranosyl)purine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194172 (5'-O-tritylinosine | CHEMBL386148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194189 (9-(5-O-trityl-beta-D-ribofuranosyl)purine | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194177 (1-methyl-5'-O-tritylinosine | CHEMBL212853) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194187 (5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50349611 (CHEMBL1808951 | CHEMBL2216905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR2 expressed in Sf9 insect cells assessed as inhibition of poly(Glu,Tyr) 4:1 substrate phosphorylation by radiome... | Eur J Med Chem 58: 160-70 (2012) Article DOI: 10.1016/j.ejmech.2012.10.001 BindingDB Entry DOI: 10.7270/Q2J38TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50194188 (5'-O-trityl-2'deoxyinosine | CHEMBL209655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of human TPase in presence of 100 uM thymidine | J Med Chem 49: 5562-70 (2006) Article DOI: 10.1021/jm0605379 BindingDB Entry DOI: 10.7270/Q2WD4061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |