 Found 501 hits with Last Name = 'mishra' and Initial = 's'
Found 501 hits with Last Name = 'mishra' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50094037
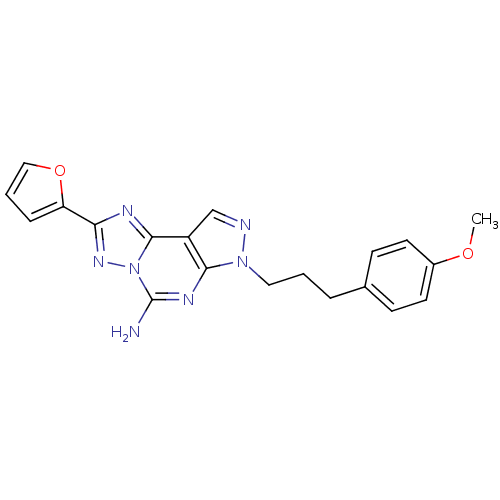
(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342512
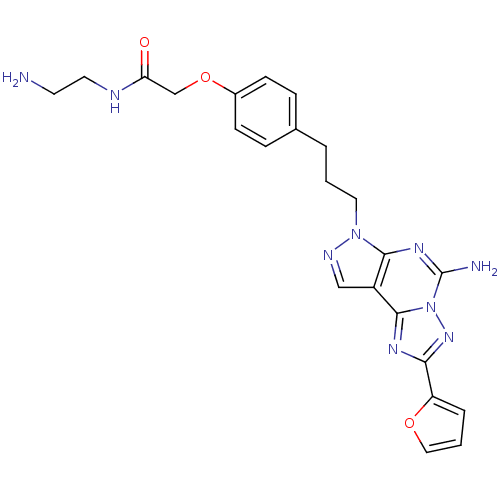
(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES NCCNC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C23H25N9O3/c24-9-10-26-19(33)14-35-16-7-5-15(6-8-16)3-1-11-31-21-17(13-27-31)22-28-20(18-4-2-12-34-18)30-32(22)23(25)29-21/h2,4-8,12-13H,1,3,9-11,14,24H2,(H2,25,29)(H,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342511
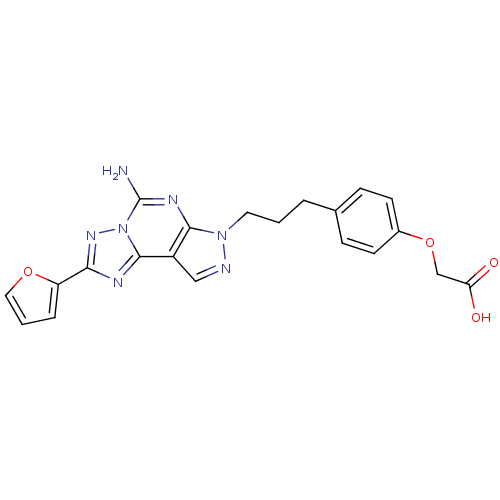
(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(O)=O)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H19N7O4/c22-21-25-19-15(20-24-18(26-28(20)21)16-4-2-10-31-16)11-23-27(19)9-1-3-13-5-7-14(8-6-13)32-12-17(29)30/h2,4-8,10-11H,1,3,9,12H2,(H2,22,25)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342516
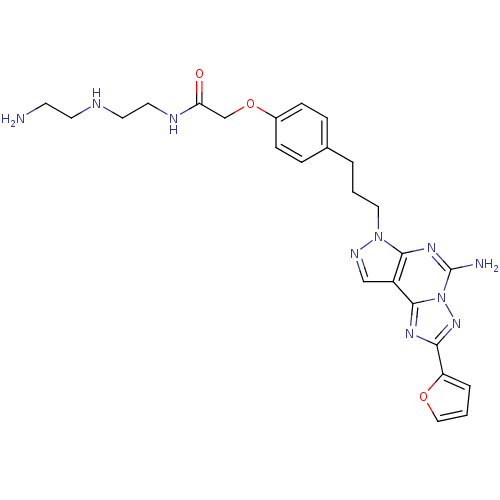
(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES NCCNCCNC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C25H30N10O3/c26-9-10-28-11-12-29-21(36)16-38-18-7-5-17(6-8-18)3-1-13-34-23-19(15-30-34)24-31-22(20-4-2-14-37-20)33-35(24)25(27)32-23/h2,4-8,14-15,28H,1,3,9-13,16,26H2,(H2,27,32)(H,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342518
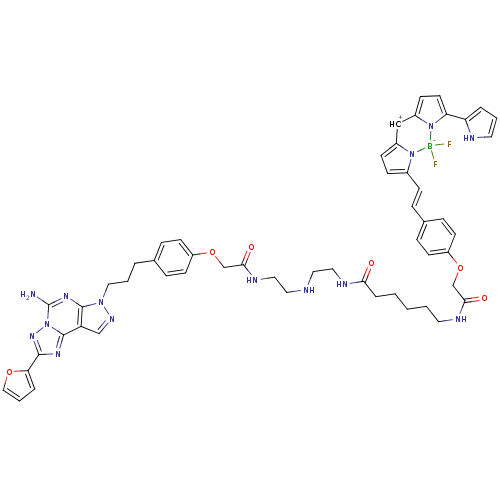
((Z)-3-(4-((1-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyra...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNCCNC(=O)CCCCCNC(=O)COc4ccc([CH+]\C=c5\ccc6=Cc7ccc(-c8ccc[nH]8)n7[B-](F)(F)n56)cc4)cc3)ncc2c2nc(nn12)-c1ccco1 |t:44| Show InChI InChI=1S/C54H57BF2N14O6/c56-55(57)69-39(17-18-40(69)33-41-19-24-46(70(41)55)45-8-4-26-60-45)16-11-38-14-22-43(23-15-38)76-35-49(73)61-25-3-1-2-10-48(72)62-29-27-59-28-30-63-50(74)36-77-42-20-12-37(13-21-42)7-5-31-68-52-44(34-64-68)53-65-51(47-9-6-32-75-47)67-71(53)54(58)66-52/h4,6,8-9,11-24,26,32-34,59-60H,1-3,5,7,10,25,27-31,35-36H2,(H2,58,66)(H,61,73)(H,62,72)(H,63,74)/b39-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50064700
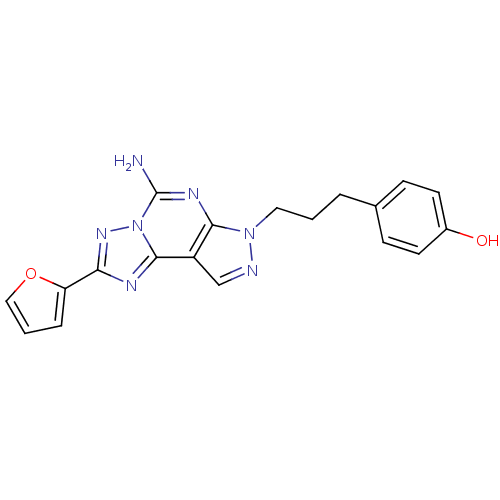
(4-(3-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1,...)Show SMILES Nc1nc2n(CCCc3ccc(O)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H17N7O2/c20-19-23-17-14(18-22-16(24-26(18)19)15-4-2-10-28-15)11-21-25(17)9-1-3-12-5-7-13(27)8-6-12/h2,4-8,10-11,27H,1,3,9H2,(H2,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342509

(7-(3-(4-(3-Fluoropropoxy)phenyl)propyl)-2-(furan-2...)Show SMILES Nc1nc2n(CCCc3ccc(OCCCF)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22FN7O2/c23-10-3-13-31-16-8-6-15(7-9-16)4-1-11-29-20-17(14-25-29)21-26-19(18-5-2-12-32-18)28-30(21)22(24)27-20/h2,5-9,12,14H,1,3-4,10-11,13H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342513
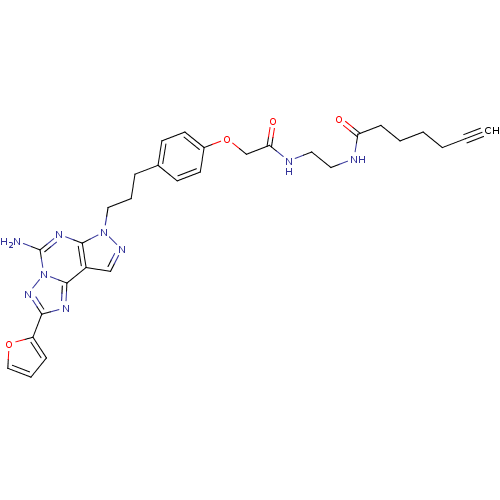
(CHEMBL1771811 | N-(2-(2-(4-(3-(5-Amino-2-(furan-2-...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNC(=O)CCCCC#C)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C30H33N9O4/c1-2-3-4-5-10-25(40)32-15-16-33-26(41)20-43-22-13-11-21(12-14-22)8-6-17-38-28-23(19-34-38)29-35-27(24-9-7-18-42-24)37-39(29)30(31)36-28/h1,7,9,11-14,18-19H,3-6,8,10,15-17,20H2,(H2,31,36)(H,32,40)(H,33,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342510
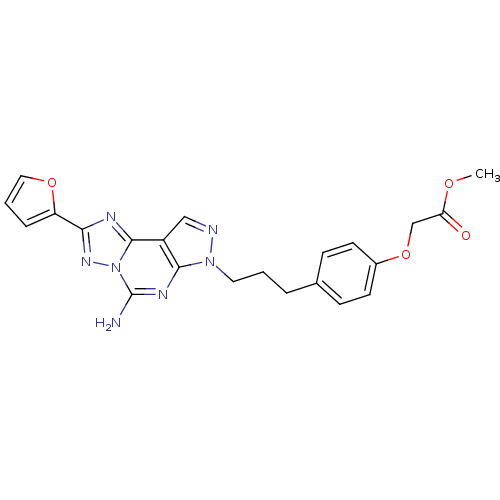
(CHEMBL1771805 | Methyl2-(4-(3-(5-amino-2-(furan-2-...)Show SMILES COC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C22H21N7O4/c1-31-18(30)13-33-15-8-6-14(7-9-15)4-2-10-28-20-16(12-24-28)21-25-19(17-5-3-11-32-17)27-29(21)22(23)26-20/h3,5-9,11-12H,2,4,10,13H2,1H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50587759
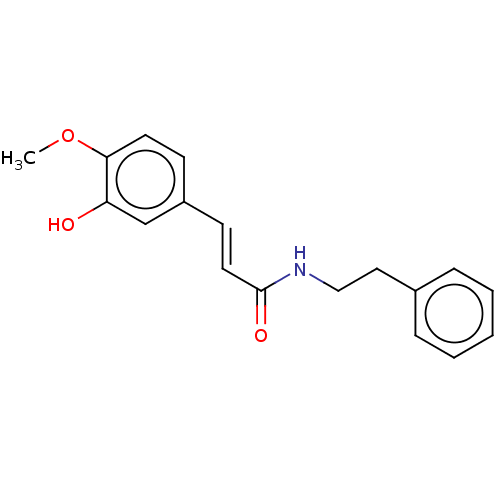
(CHEMBL4163108) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342519
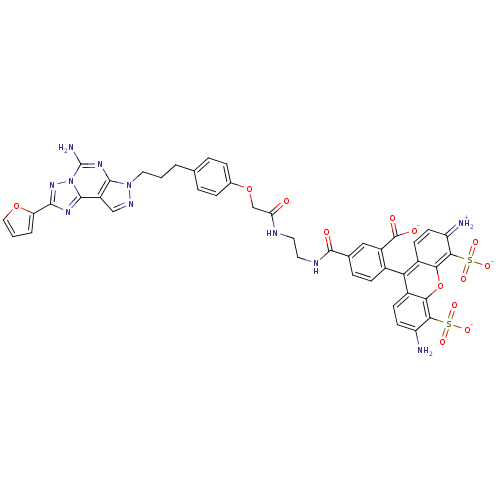
(CHEMBL1771809 | triethylammonium 5-(2-(2-(4-(3-(5-...)Show SMILES Nc1ccc2c(-c3ccc(cc3C([O-])=O)C(=O)NCCNC(=O)COc3ccc(CCCn4ncc5c4nc(N)n4nc(nc54)-c4ccco4)cc3)c3ccc(=[NH2+])c(c3oc2c1S([O-])(=O)=O)S([O-])(=O)=O |(9.9,8.23,;8.57,7.44,;7.23,8.2,;5.91,7.42,;5.93,5.89,;4.6,5.12,;3.27,5.88,;3.26,7.43,;1.92,8.19,;.6,7.41,;.6,5.87,;1.93,5.1,;1.15,3.78,;1.91,2.44,;-.39,3.79,;-.74,8.17,;-.75,9.71,;-2.07,7.4,;-3.41,8.16,;-4.74,7.39,;-6.08,8.16,;-7.41,7.38,;-7.41,5.84,;-8.75,8.15,;-10.08,7.37,;-10.08,5.83,;-11.41,5.06,;-11.41,3.52,;-10.07,2.74,;-10.07,1.2,;-8.74,.43,;-8.74,-1.11,;-7.41,-1.88,;-7.24,-3.41,;-5.73,-3.73,;-4.97,-2.39,;-5.99,-1.25,;-5.52,.21,;-4.01,.52,;-3.53,1.98,;-2.99,-.62,;-1.45,-.63,;-.98,-2.09,;-2.23,-2.99,;-3.47,-2.08,;.49,-2.57,;.96,-4.04,;2.5,-4.05,;2.98,-2.58,;1.74,-1.67,;-8.74,3.52,;-8.74,5.07,;4.62,3.58,;3.29,2.81,;3.29,1.27,;4.63,.51,;4.64,-1.03,;5.95,1.28,;5.94,2.81,;7.28,3.58,;7.26,5.13,;8.58,5.91,;9.93,5.15,;9.94,3.61,;11.01,6.25,;11.41,4.76,;7.29,.52,;7.29,-1.02,;8.05,1.86,;8.77,.13,)| Show InChI InChI=1S/C44H37N11O13S2/c45-30-13-11-26-34(27-12-14-31(46)38(70(63,64)65)36(27)68-35(26)37(30)69(60,61)62)25-10-7-23(19-28(25)43(58)59)42(57)49-16-15-48-33(56)21-67-24-8-5-22(6-9-24)3-1-17-54-40-29(20-50-54)41-51-39(32-4-2-18-66-32)53-55(41)44(47)52-40/h2,4-14,18-20,45H,1,3,15-17,21,46H2,(H2,47,52)(H,48,56)(H,49,57)(H,58,59)(H,60,61,62)(H,63,64,65)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342515
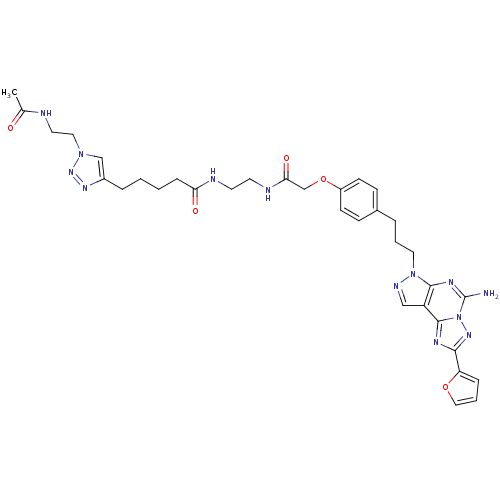
(5-(1-(2-Acetamidoethyl)-1H-1,2,3-triazol-4-yl)-N-(...)Show SMILES CC(=O)NCCn1cc(CCCCC(=O)NCCNC(=O)COc2ccc(CCCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)cc2)nn1 Show InChI InChI=1S/C34H41N13O5/c1-23(48)36-16-18-45-21-25(42-44-45)7-2-3-9-29(49)37-14-15-38-30(50)22-52-26-12-10-24(11-13-26)6-4-17-46-32-27(20-39-46)33-40-31(28-8-5-19-51-28)43-47(33)34(35)41-32/h5,8,10-13,19-21H,2-4,6-7,9,14-18,22H2,1H3,(H2,35,41)(H,36,48)(H,37,49)(H,38,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50342515

(5-(1-(2-Acetamidoethyl)-1H-1,2,3-triazol-4-yl)-N-(...)Show SMILES CC(=O)NCCn1cc(CCCCC(=O)NCCNC(=O)COc2ccc(CCCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)cc2)nn1 Show InChI InChI=1S/C34H41N13O5/c1-23(48)36-16-18-45-21-25(42-44-45)7-2-3-9-29(49)37-14-15-38-30(50)22-52-26-12-10-24(11-13-26)6-4-17-46-32-27(20-39-46)33-40-31(28-8-5-19-51-28)43-47(33)34(35)41-32/h5,8,10-13,19-21H,2-4,6-7,9,14-18,22H2,1H3,(H2,35,41)(H,36,48)(H,37,49)(H,38,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342514

(CHEMBL1771812 | N-(2-(2-(4-(3-(5-Amino-2-(furan-2-...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNC(=O)CCCCc4cn(CCCCCCC(=O)CCOCCOCCOCCOCCNC(=O)CCCC[C@@H]5SC[C@@H]6NC(=O)N[C@H]56)nn4)cc3)ncc2c2nc(nn12)-c1ccco1 |r| Show InChI InChI=1S/C57H81N15O11S/c58-56-66-54-45(55-65-53(68-72(55)56)47-14-10-28-82-47)37-62-71(54)27-9-11-41-18-20-44(21-19-41)83-39-51(76)60-24-23-59-49(74)16-6-4-12-42-38-70(69-67-42)26-8-2-1-3-13-43(73)22-29-78-31-33-80-35-36-81-34-32-79-30-25-61-50(75)17-7-5-15-48-52-46(40-84-48)63-57(77)64-52/h10,14,18-21,28,37-38,46,48,52H,1-9,11-13,15-17,22-27,29-36,39-40H2,(H2,58,66)(H,59,74)(H,60,76)(H,61,75)(H2,63,64,77)/t46-,48-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50342517
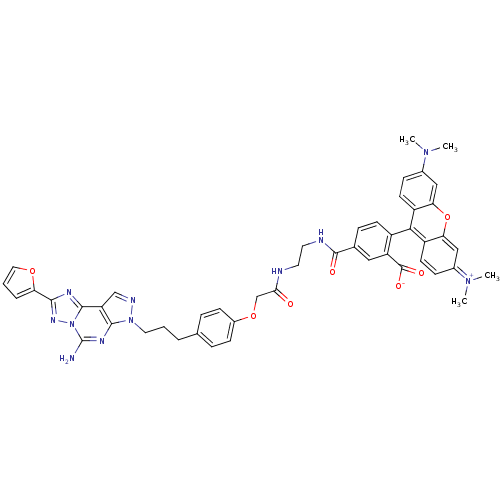
(5-((2-(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo...)Show SMILES CN(C)c1ccc2c(-c3ccc(cc3C([O-])=O)C(=O)NCCNC(=O)COc3ccc(CCCn4ncc5c4nc(N)n4nc(nc54)-c4ccco4)cc3)c3ccc(cc3oc2c1)=[N+](C)C |(40.33,-23.99,;38.99,-23.23,;38.97,-21.69,;37.66,-24.02,;36.31,-23.26,;35,-24.04,;35.01,-25.57,;33.69,-26.35,;32.35,-25.58,;32.34,-24.04,;31.01,-23.28,;29.68,-24.05,;29.68,-25.59,;31.02,-26.36,;30.24,-27.68,;30.99,-29.03,;28.7,-27.67,;28.35,-23.29,;28.34,-21.75,;27.01,-24.06,;25.68,-23.3,;24.34,-24.07,;23.01,-23.31,;21.67,-24.08,;21.68,-25.62,;20.34,-23.31,;19.01,-24.09,;19.01,-25.63,;17.68,-26.4,;17.68,-27.95,;19.01,-28.72,;19.01,-30.26,;20.35,-31.03,;20.35,-32.57,;21.68,-33.34,;21.85,-34.87,;23.35,-35.19,;24.12,-33.85,;23.09,-32.71,;23.57,-31.25,;25.08,-30.94,;25.56,-29.48,;26.1,-32.09,;27.63,-32.09,;28.1,-33.55,;26.86,-34.45,;25.62,-33.54,;29.57,-34.04,;29.39,-35.57,;30.79,-36.21,;31.84,-35.08,;31.08,-33.73,;20.35,-27.94,;20.35,-26.39,;33.7,-27.89,;32.38,-28.65,;32.38,-30.19,;33.72,-30.95,;35.04,-30.18,;35.03,-28.65,;36.36,-27.88,;36.35,-26.33,;37.67,-25.55,;33.72,-32.49,;35.06,-33.26,;32.39,-33.27,)| Show InChI InChI=1S/C48H45N11O7/c1-56(2)30-12-17-34-39(24-30)66-40-25-31(57(3)4)13-18-35(40)42(34)33-16-11-29(23-36(33)47(62)63)46(61)51-20-19-50-41(60)27-65-32-14-9-28(10-15-32)7-5-21-58-44-37(26-52-58)45-53-43(38-8-6-22-64-38)55-59(45)48(49)54-44/h6,8-18,22-26H,5,7,19-21,27H2,1-4H3,(H4-,49,50,51,52,53,54,55,60,61,62,63) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in HEK293 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342509

(7-(3-(4-(3-Fluoropropoxy)phenyl)propyl)-2-(furan-2...)Show SMILES Nc1nc2n(CCCc3ccc(OCCCF)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22FN7O2/c23-10-3-13-31-16-8-6-15(7-9-16)4-1-11-29-20-17(14-25-29)21-26-19(18-5-2-12-32-18)28-30(21)22(24)27-20/h2,5-9,12,14H,1,3-4,10-11,13H2,(H2,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50342514

(CHEMBL1771812 | N-(2-(2-(4-(3-(5-Amino-2-(furan-2-...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNC(=O)CCCCc4cn(CCCCCCC(=O)CCOCCOCCOCCOCCNC(=O)CCCC[C@@H]5SC[C@@H]6NC(=O)N[C@H]56)nn4)cc3)ncc2c2nc(nn12)-c1ccco1 |r| Show InChI InChI=1S/C57H81N15O11S/c58-56-66-54-45(55-65-53(68-72(55)56)47-14-10-28-82-47)37-62-71(54)27-9-11-41-18-20-44(21-19-41)83-39-51(76)60-24-23-59-49(74)16-6-4-12-42-38-70(69-67-42)26-8-2-1-3-13-43(73)22-29-78-31-33-80-35-36-81-34-32-79-30-25-61-50(75)17-7-5-15-48-52-46(40-84-48)63-57(77)64-52/h10,14,18-21,28,37-38,46,48,52H,1-9,11-13,15-17,22-27,29-36,39-40H2,(H2,58,66)(H,59,74)(H,60,76)(H,61,75)(H2,63,64,77)/t46-,48-,52-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342513

(CHEMBL1771811 | N-(2-(2-(4-(3-(5-Amino-2-(furan-2-...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNC(=O)CCCCC#C)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C30H33N9O4/c1-2-3-4-5-10-25(40)32-15-16-33-26(41)20-43-22-13-11-21(12-14-22)8-6-17-38-28-23(19-34-38)29-35-27(24-9-7-18-42-24)37-39(29)30(31)36-28/h1,7,9,11-14,18-19H,3-6,8,10,15-17,20H2,(H2,31,36)(H,32,40)(H,33,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342512

(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES NCCNC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C23H25N9O3/c24-9-10-26-19(33)14-35-16-7-5-15(6-8-16)3-1-11-31-21-17(13-27-31)22-28-20(18-4-2-12-34-18)30-32(22)23(25)29-21/h2,4-8,12-13H,1,3,9-11,14,24H2,(H2,25,29)(H,26,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50342513

(CHEMBL1771811 | N-(2-(2-(4-(3-(5-Amino-2-(furan-2-...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNC(=O)CCCCC#C)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C30H33N9O4/c1-2-3-4-5-10-25(40)32-15-16-33-26(41)20-43-22-13-11-21(12-14-22)8-6-17-38-28-23(19-34-38)29-35-27(24-9-7-18-42-24)37-39(29)30(31)36-28/h1,7,9,11-14,18-19H,3-6,8,10,15-17,20H2,(H2,31,36)(H,32,40)(H,33,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342514

(CHEMBL1771812 | N-(2-(2-(4-(3-(5-Amino-2-(furan-2-...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(=O)NCCNC(=O)CCCCc4cn(CCCCCCC(=O)CCOCCOCCOCCOCCNC(=O)CCCC[C@@H]5SC[C@@H]6NC(=O)N[C@H]56)nn4)cc3)ncc2c2nc(nn12)-c1ccco1 |r| Show InChI InChI=1S/C57H81N15O11S/c58-56-66-54-45(55-65-53(68-72(55)56)47-14-10-28-82-47)37-62-71(54)27-9-11-41-18-20-44(21-19-41)83-39-51(76)60-24-23-59-49(74)16-6-4-12-42-38-70(69-67-42)26-8-2-1-3-13-43(73)22-29-78-31-33-80-35-36-81-34-32-79-30-25-61-50(75)17-7-5-15-48-52-46(40-84-48)63-57(77)64-52/h10,14,18-21,28,37-38,46,48,52H,1-9,11-13,15-17,22-27,29-36,39-40H2,(H2,58,66)(H,59,74)(H,60,76)(H,61,75)(H2,63,64,77)/t46-,48-,52-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50342509

(7-(3-(4-(3-Fluoropropoxy)phenyl)propyl)-2-(furan-2...)Show SMILES Nc1nc2n(CCCc3ccc(OCCCF)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22FN7O2/c23-10-3-13-31-16-8-6-15(7-9-16)4-1-11-29-20-17(14-25-29)21-26-19(18-5-2-12-32-18)28-30(21)22(24)27-20/h2,5-9,12,14H,1,3-4,10-11,13H2,(H2,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342515

(5-(1-(2-Acetamidoethyl)-1H-1,2,3-triazol-4-yl)-N-(...)Show SMILES CC(=O)NCCn1cc(CCCCC(=O)NCCNC(=O)COc2ccc(CCCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)cc2)nn1 Show InChI InChI=1S/C34H41N13O5/c1-23(48)36-16-18-45-21-25(42-44-45)7-2-3-9-29(49)37-14-15-38-30(50)22-52-26-12-10-24(11-13-26)6-4-17-46-32-27(20-39-46)33-40-31(28-8-5-19-51-28)43-47(33)34(35)41-32/h5,8,10-13,19-21H,2-4,6-7,9,14-18,22H2,1H3,(H2,35,41)(H,36,48)(H,37,49)(H,38,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342511

(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES Nc1nc2n(CCCc3ccc(OCC(O)=O)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H19N7O4/c22-21-25-19-15(20-24-18(26-28(20)21)16-4-2-10-31-16)11-23-27(19)9-1-3-13-5-7-14(8-6-13)32-12-17(29)30/h2,4-8,10-11H,1,3,9,12H2,(H2,22,25)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50587759

(CHEMBL4163108) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50342516

(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES NCCNCCNC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C25H30N10O3/c26-9-10-28-11-12-29-21(36)16-38-18-7-5-17(6-8-18)3-1-13-34-23-19(15-30-34)24-31-22(20-4-2-14-37-20)33-35(24)25(27)32-23/h2,4-8,14-15,28H,1,3,9-13,16,26H2,(H2,27,32)(H,29,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50342512

(2-(4-(3-(5-Amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e]...)Show SMILES NCCNC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C23H25N9O3/c24-9-10-26-19(33)14-35-16-7-5-15(6-8-16)3-1-11-31-21-17(13-27-31)22-28-20(18-4-2-12-34-18)30-32(22)23(25)29-21/h2,4-8,12-13H,1,3,9-11,14,24H2,(H2,25,29)(H,26,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50064700

(4-(3-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1,...)Show SMILES Nc1nc2n(CCCc3ccc(O)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H17N7O2/c20-19-23-17-14(18-22-16(24-26(18)19)15-4-2-10-28-15)11-21-25(17)9-1-3-12-5-7-13(27)8-6-12/h2,4-8,10-11,27H,1,3,9H2,(H2,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50342510

(CHEMBL1771805 | Methyl2-(4-(3-(5-amino-2-(furan-2-...)Show SMILES COC(=O)COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C22H21N7O4/c1-31-18(30)13-33-15-8-6-14(7-9-15)4-2-10-28-20-16(12-24-28)21-25-19(17-5-3-11-32-17)27-29(21)22(23)26-20/h3,5-9,11-12H,2,4,10,13H2,1H3,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 2740-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.082
BindingDB Entry DOI: 10.7270/Q2RV0P0B |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587744

(CHEMBL5177936)Show SMILES COc1cc(\C=C\C(=O)OCCCCCN(C)Cc2cccc(OC(=O)N(C)C)c2)ccc1O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50587756

(CHEMBL5206601)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3ccc(F)cc3)cc2)cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50587755
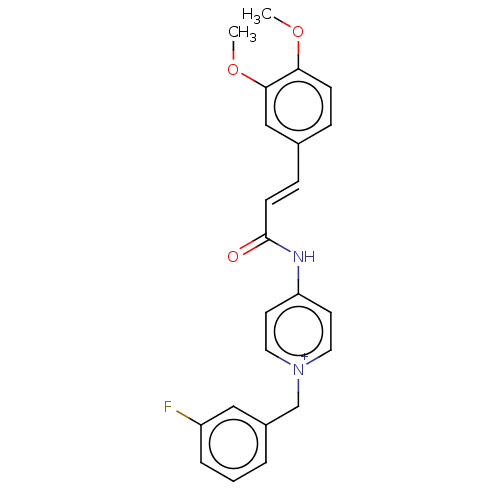
(CHEMBL5171929)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3cccc(F)c3)cc2)cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50450704
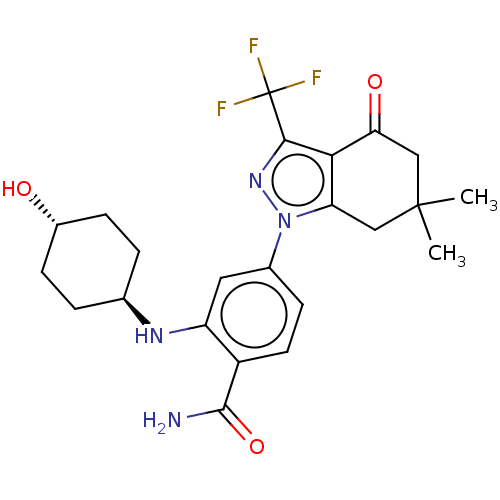
(CHEMBL560895 | SNX-2112)Show SMILES CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@H](O)CC3)c2)C(F)(F)F)C(=O)C1 |r,wU:18.18,wD:21.22,(-2.35,-2,;-2.38,-.77,;-3.43,-.12,;-1.03,-1.55,;.3,-.77,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,;2.24,2.7,;3.44,2.95,;1.41,3.61,;2.62,3.87,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,)| Show InChI InChI=1S/C23H27F3N4O3/c1-22(2)10-17-19(18(32)11-22)20(23(24,25)26)29-30(17)13-5-8-15(21(27)33)16(9-13)28-12-3-6-14(31)7-4-12/h5,8-9,12,14,28,31H,3-4,6-7,10-11H2,1-2H3,(H2,27,33)/t12-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-beta incubated for 2 hrs by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01700
BindingDB Entry DOI: 10.7270/Q2125X9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587756

(CHEMBL5206601)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3ccc(F)cc3)cc2)cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587755

(CHEMBL5171929)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3cccc(F)c3)cc2)cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50003868
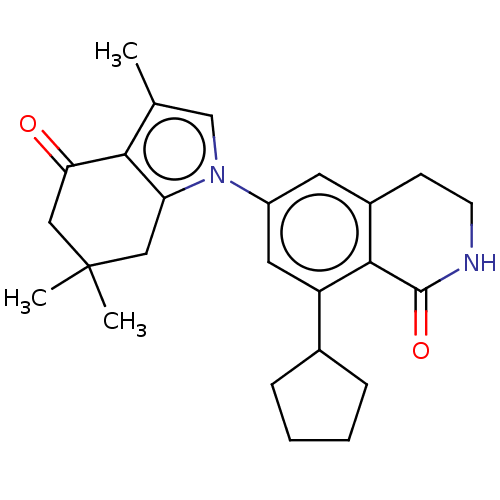
(CHEMBL3235353)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1cc2CCNC(=O)c2c(c1)C1CCCC1 Show InChI InChI=1S/C25H30N2O2/c1-15-14-27(20-12-25(2,3)13-21(28)22(15)20)18-10-17-8-9-26-24(29)23(17)19(11-18)16-6-4-5-7-16/h10-11,14,16H,4-9,12-13H2,1-3H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-alpha incubated for 2 hrs by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01700
BindingDB Entry DOI: 10.7270/Q2125X9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50003868

(CHEMBL3235353)Show SMILES Cc1cn(c2CC(C)(C)CC(=O)c12)-c1cc2CCNC(=O)c2c(c1)C1CCCC1 Show InChI InChI=1S/C25H30N2O2/c1-15-14-27(20-12-25(2,3)13-21(28)22(15)20)18-10-17-8-9-26-24(29)23(17)19(11-18)16-6-4-5-7-16/h10-11,14,16H,4-9,12-13H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-beta incubated for 2 hrs by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01700
BindingDB Entry DOI: 10.7270/Q2125X9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50450704

(CHEMBL560895 | SNX-2112)Show SMILES CC1(C)Cc2c(c(nn2-c2ccc(C(N)=O)c(N[C@H]3CC[C@H](O)CC3)c2)C(F)(F)F)C(=O)C1 |r,wU:18.18,wD:21.22,(-2.35,-2,;-2.38,-.77,;-3.43,-.12,;-1.03,-1.55,;.3,-.77,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;2.98,-5.68,;3.35,-7.18,;4.53,-7.52,;2.46,-8.03,;4.09,-4.62,;5.57,-5.04,;6.68,-3.97,;6.3,-2.47,;7.41,-1.4,;8.89,-1.82,;9.77,-.96,;9.27,-3.32,;8.16,-4.39,;3.72,-3.12,;2.24,2.7,;3.44,2.95,;1.41,3.61,;2.62,3.87,;-1.03,1.55,;-1.03,2.79,;-2.38,.77,)| Show InChI InChI=1S/C23H27F3N4O3/c1-22(2)10-17-19(18(32)11-22)20(23(24,25)26)29-30(17)13-5-8-15(21(27)33)16(9-13)28-12-3-6-14(31)7-4-12/h5,8-9,12,14,28,31H,3-4,6-7,10-11H2,1-2H3,(H2,27,33)/t12-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-alpha incubated for 2 hrs by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01700
BindingDB Entry DOI: 10.7270/Q2125X9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587756

(CHEMBL5206601)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3ccc(F)cc3)cc2)cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50510839
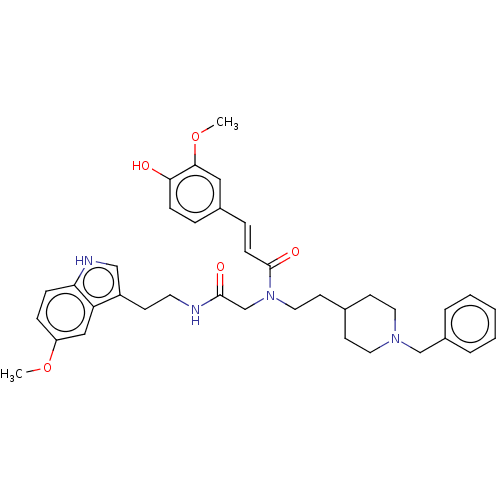
(CHEMBL4536838)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CN(CCC3CCN(Cc4ccccc4)CC3)C(=O)\C=C\c3ccc(O)c(OC)c3)c2c1 Show InChI InChI=1S/C37H44N4O5/c1-45-31-10-11-33-32(23-31)30(24-39-33)14-18-38-36(43)26-41(37(44)13-9-28-8-12-34(42)35(22-28)46-2)21-17-27-15-19-40(20-16-27)25-29-6-4-3-5-7-29/h3-13,22-24,27,39,42H,14-21,25-26H2,1-2H3,(H,38,43)/b13-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50587756

(CHEMBL5206601)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3ccc(F)cc3)cc2)cc1OC | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50362851
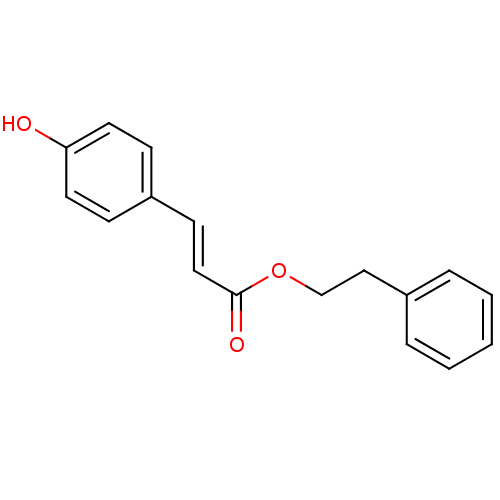
(CHEMBL1940390)Show InChI InChI=1S/C17H16O3/c18-16-9-6-15(7-10-16)8-11-17(19)20-13-12-14-4-2-1-3-5-14/h1-11,18H,12-13H2/b11-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587755

(CHEMBL5171929)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3cccc(F)c3)cc2)cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50587744

(CHEMBL5177936)Show SMILES COc1cc(\C=C\C(=O)OCCCCCN(C)Cc2cccc(OC(=O)N(C)C)c2)ccc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50587755

(CHEMBL5171929)Show SMILES [Br-].COc1ccc(\C=C\C(=O)Nc2cc[n+](Cc3cccc(F)c3)cc2)cc1OC | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510850
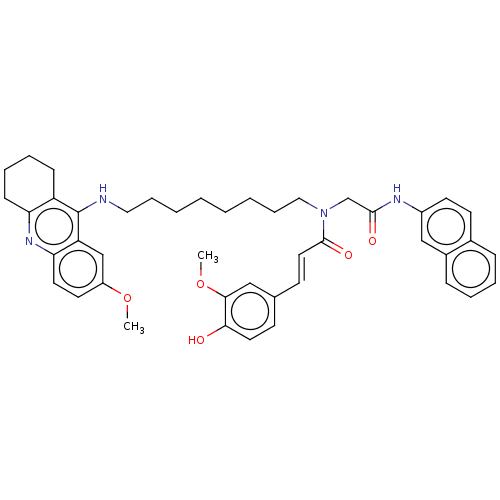
(CHEMBL4552675)Show SMILES COc1ccc2nc3CCCCc3c(NCCCCCCCCN(CC(=O)Nc3ccc4ccccc4c3)C(=O)\C=C\c3ccc(O)c(OC)c3)c2c1 Show InChI InChI=1S/C44H50N4O5/c1-52-35-21-22-39-37(29-35)44(36-15-9-10-16-38(36)47-39)45-25-11-5-3-4-6-12-26-48(43(51)24-18-31-17-23-40(49)41(27-31)53-2)30-42(50)46-34-20-19-32-13-7-8-14-33(32)28-34/h7-8,13-14,17-24,27-29,49H,3-6,9-12,15-16,25-26,30H2,1-2H3,(H,45,47)(H,46,50)/b24-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113278
BindingDB Entry DOI: 10.7270/Q2PR80XJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50557204

(CHEMBL4740377)Show SMILES CCCC1Cc2cc(cc(N[C@H]3CC[C@H](O)CC3)c2C(=O)N1)-n1nc(C)c2c1CC(C)(C)CC2=O |r,wU:11.10,wD:14.14,(16.82,-13.5,;15.5,-12.72,;14.16,-13.48,;12.83,-12.7,;12.84,-11.16,;11.52,-10.38,;11.52,-8.84,;10.18,-8.07,;8.85,-8.84,;8.87,-10.37,;7.53,-11.13,;6.19,-10.37,;4.86,-11.13,;3.53,-10.37,;3.53,-8.83,;2.2,-8.06,;4.86,-8.05,;6.19,-8.83,;10.19,-11.15,;10.18,-12.68,;8.85,-13.45,;11.5,-13.45,;10.18,-6.52,;8.93,-5.62,;9.41,-4.15,;8.51,-2.9,;10.95,-4.15,;11.43,-5.63,;12.94,-5.95,;13.98,-4.81,;15.12,-5.84,;15.45,-4.34,;13.51,-3.33,;11.99,-3,;11.52,-1.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-beta incubated for 2 hrs by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01700
BindingDB Entry DOI: 10.7270/Q2125X9Q |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50610092
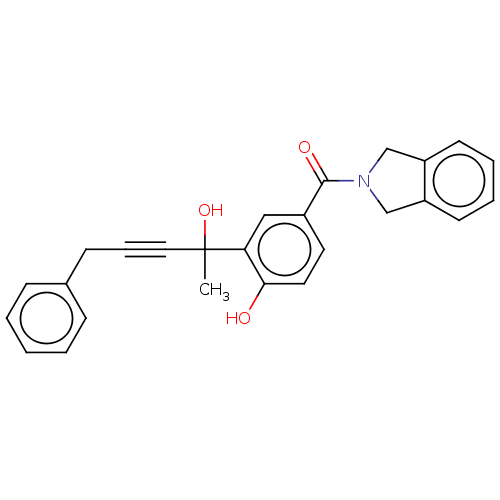
(CHEMBL5276395)Show SMILES CC(O)(C#CCc1ccccc1)c1cc(ccc1O)C(=O)N1Cc2ccccc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50557190

(CHEMBL4796520)Show SMILES CCCc1cc2cc(cc(N[C@H]3CC[C@H](O)CC3)c2c(=O)[nH]1)-n1nc(C)c2c1CC(C)(C)CC2=O |r,wU:11.10,wD:14.14,(13.84,-31.1,;12.51,-30.32,;12.52,-28.78,;11.2,-28,;11.21,-26.47,;9.88,-25.69,;9.89,-24.14,;8.55,-23.37,;7.22,-24.14,;7.24,-25.68,;5.9,-26.44,;4.56,-25.68,;3.23,-26.44,;1.9,-25.68,;1.9,-24.14,;.56,-23.37,;3.23,-23.36,;4.56,-24.14,;8.56,-26.45,;8.55,-27.98,;7.22,-28.76,;9.86,-28.76,;8.55,-21.83,;7.3,-20.92,;7.78,-19.46,;6.88,-18.21,;9.32,-19.45,;9.79,-20.93,;11.3,-21.26,;12.35,-20.11,;13.49,-21.15,;13.82,-19.65,;11.88,-18.64,;10.36,-18.3,;9.88,-16.84,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-GDA binding to human HSP90-beta incubated for 2 hrs by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01700
BindingDB Entry DOI: 10.7270/Q2125X9Q |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50610074
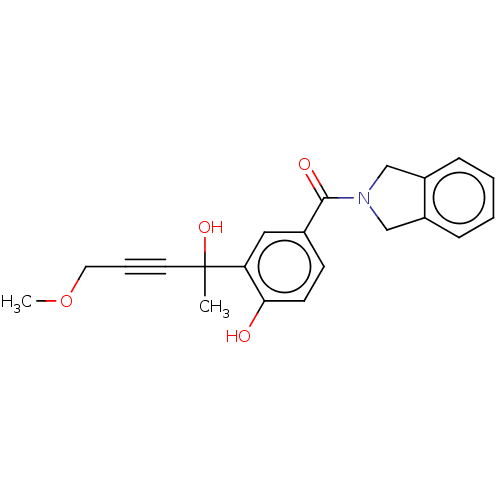
(CHEMBL5280465) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data