

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50218197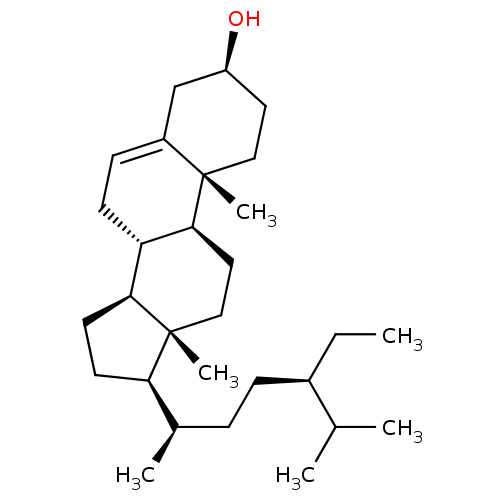 ((-)-beta-Sitosterol | (24R)-Ethylcholest-5-en-3bet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants Curated by ChEMBL | Assay Description Uncompetitive inhibition of human thrombin using T1637 as substrate by Michaelis-Menten plot analysis | J Nat Prod 81: 2521-2530 (2018) Article DOI: 10.1021/acs.jnatprod.8b00574 BindingDB Entry DOI: 10.7270/Q2BZ68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455106 (CHEMBL4216095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455107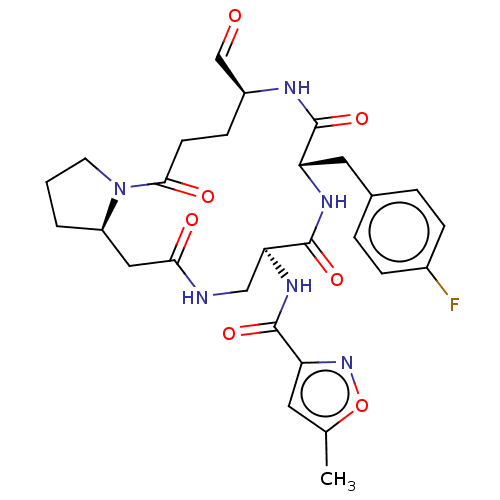 (CHEMBL4212167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455115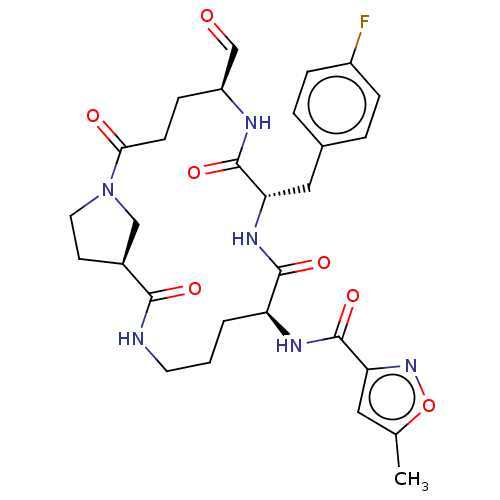 (CHEMBL4202932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Kentucky University Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase expressed in baculovirus-infected cell system using 7-methoxy-trifluoromethylcoumarin as substrate after 30... | Bioorg Med Chem 22: 126-34 (2013) Article DOI: 10.1016/j.bmc.2013.11.045 BindingDB Entry DOI: 10.7270/Q2D79CXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455105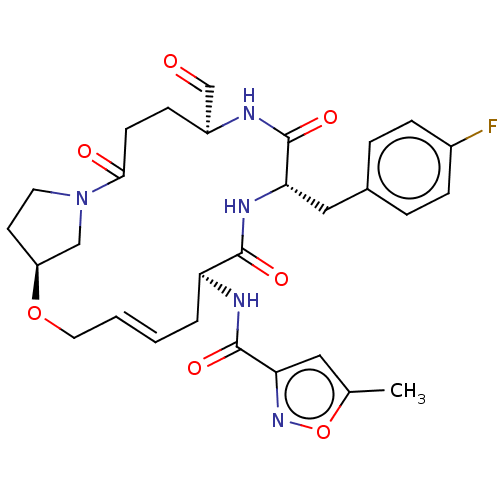 (CHEMBL4218384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455114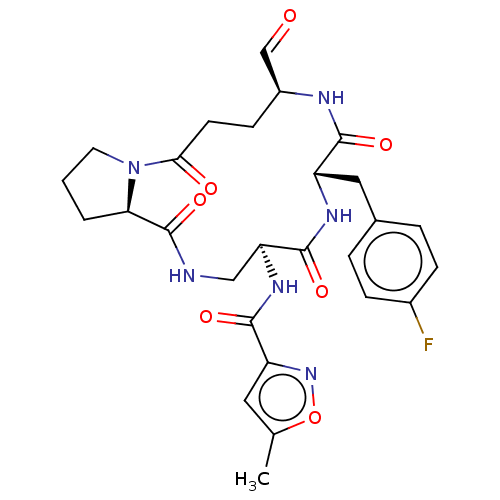 (CHEMBL4207862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455112 (CHEMBL4215964) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455113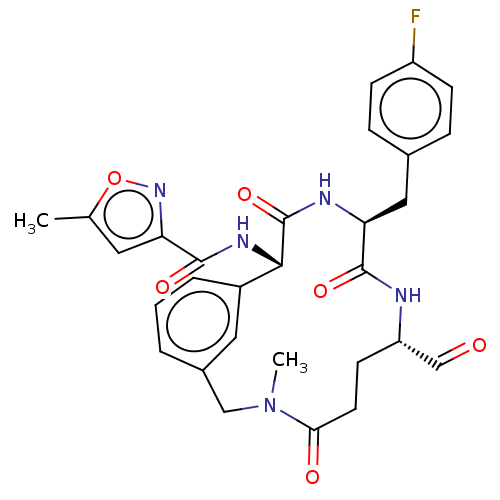 (CHEMBL4212963) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015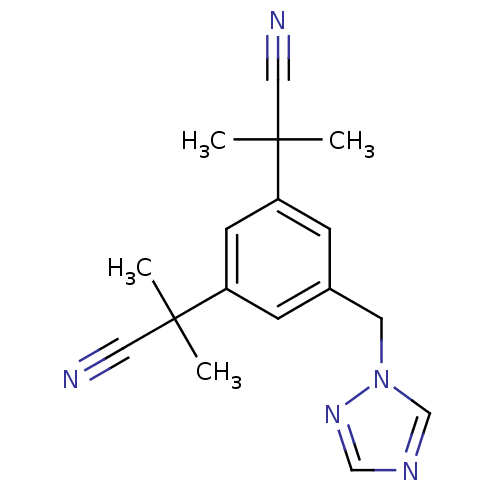 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Kentucky University Curated by ChEMBL | Assay Description Inhibition of aromatase | Bioorg Med Chem 20: 2603-13 (2012) Article DOI: 10.1016/j.bmc.2012.02.042 BindingDB Entry DOI: 10.7270/Q29S1S24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455111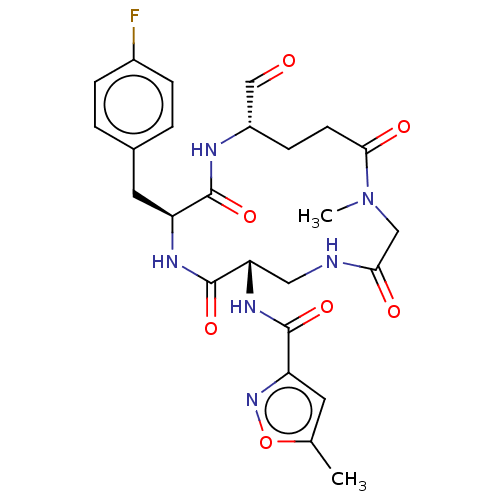 (CHEMBL4217566) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008935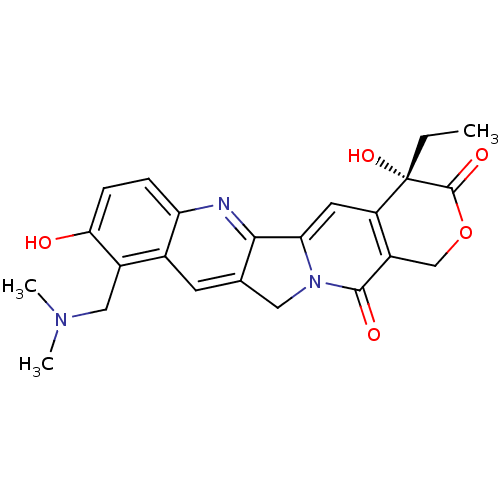 ((20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA... | J Med Chem 62: 3428-3446 (2019) Article DOI: 10.1021/acs.jmedchem.8b01938 BindingDB Entry DOI: 10.7270/Q2930XGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008923 ((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA... | J Med Chem 62: 3428-3446 (2019) Article DOI: 10.1021/acs.jmedchem.8b01938 BindingDB Entry DOI: 10.7270/Q2930XGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50510239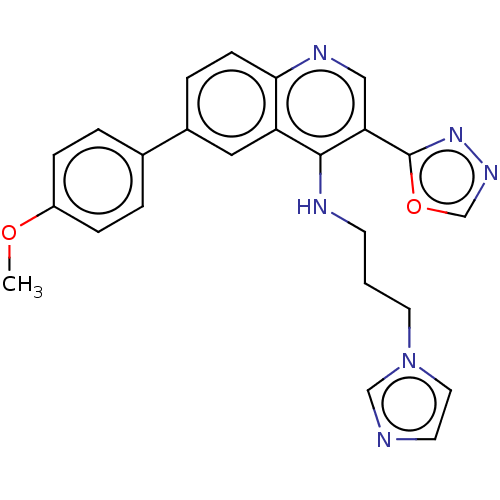 (CHEMBL4454135) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Poison activity at recombinant human TOP1 expressed in baculovirus infected Sf9 insect cells assessed as decrease in relaxed supercoiled pBS(SK+) DNA... | J Med Chem 62: 3428-3446 (2019) Article DOI: 10.1021/acs.jmedchem.8b01938 BindingDB Entry DOI: 10.7270/Q2930XGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455110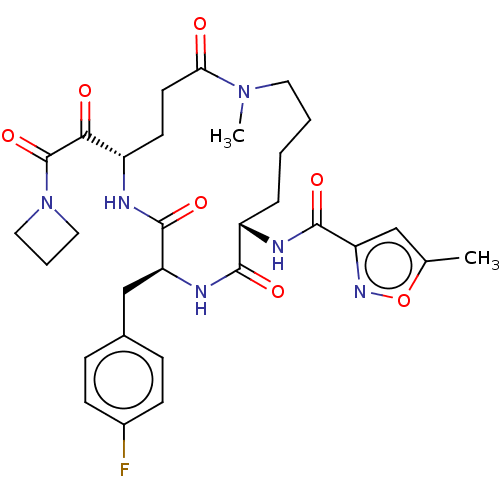 (CHEMBL4203502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558250 (CHEMBL4777850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558252 (CHEMBL4778719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558252 (CHEMBL4778719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558250 (CHEMBL4777850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558272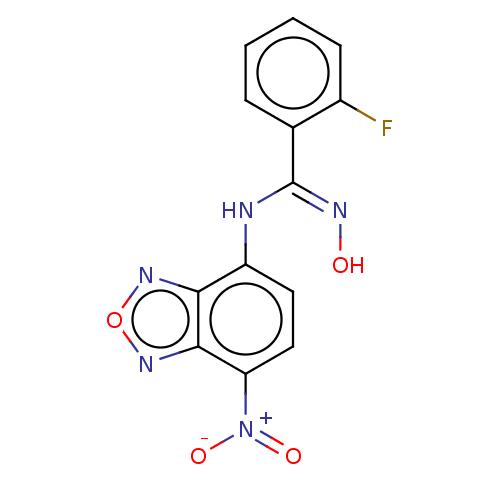 (CHEMBL4744101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558252 (CHEMBL4778719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558264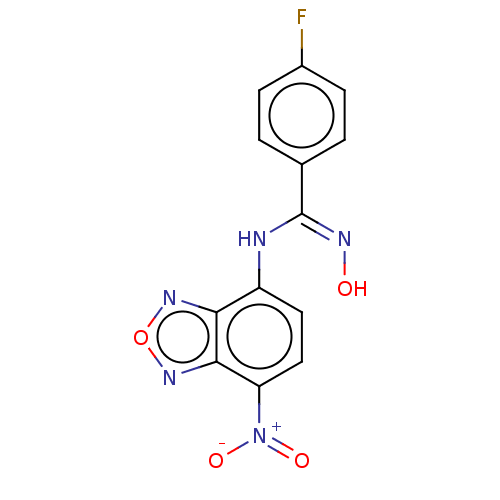 (CHEMBL4795305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455104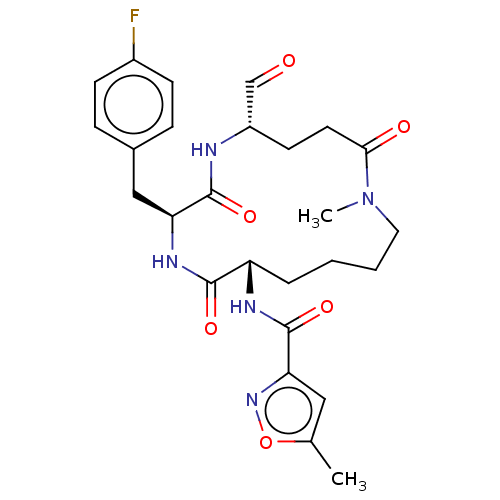 (CHEMBL4211003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558272 (CHEMBL4744101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558270 (CHEMBL4782412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558270 (CHEMBL4782412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558250 (CHEMBL4777850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558253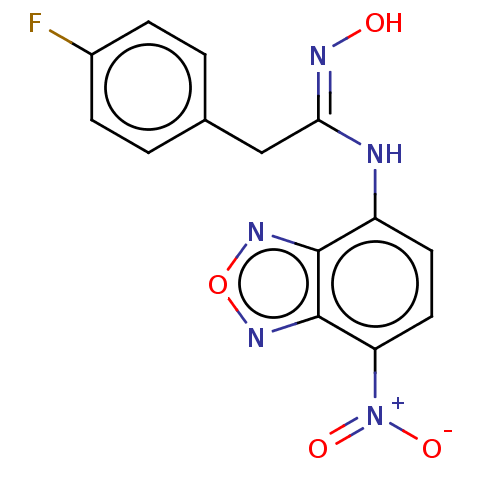 (CHEMBL4747676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558268 (CHEMBL4758067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558259 (CHEMBL4748675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558255 (CHEMBL4752806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558270 (CHEMBL4782412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558268 (CHEMBL4758067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558260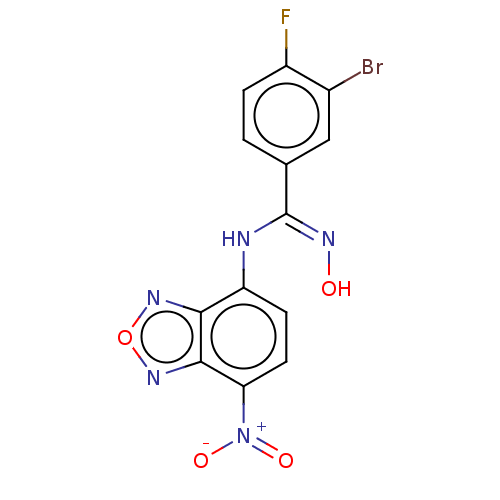 (CHEMBL4757996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558253 (CHEMBL4747676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558264 (CHEMBL4795305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558259 (CHEMBL4748675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558266 (CHEMBL4792192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558260 (CHEMBL4757996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1.5 hrs by HPLC analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558260 (CHEMBL4757996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558255 (CHEMBL4752806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558254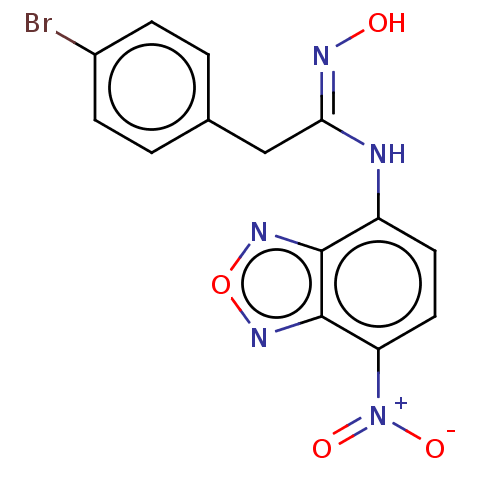 (CHEMBL4800312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558263 (CHEMBL4763146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558262 (CHEMBL4750221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558264 (CHEMBL4795305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 in IFN-gamma-induced human MDA-MB-231 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate pretrea... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558255 (CHEMBL4752806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50558256 (CHEMBL4760074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as N-formylkynurenine formation using L-tryptophan as substrate measured after 1 hr by UV-vis spectroph... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.061 BindingDB Entry DOI: 10.7270/Q2R49VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 291 total ) | Next | Last >> |