 Found 26 hits with Last Name = 'peleg' and Initial = 's'
Found 26 hits with Last Name = 'peleg' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas
Curated by ChEMBL
| Assay Description
Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assay |
J Med Chem 49: 7513-7 (2006)
Article DOI: 10.1021/jm0609925
BindingDB Entry DOI: 10.7270/Q2XG9QS7 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Displacement of 1alpha, 25-(OH)[3H]D3 from human VDR expressed in COS1 cells |
J Med Chem 50: 5824-32 (2007)
Article DOI: 10.1021/jm070882d
BindingDB Entry DOI: 10.7270/Q2NK3DSS |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925
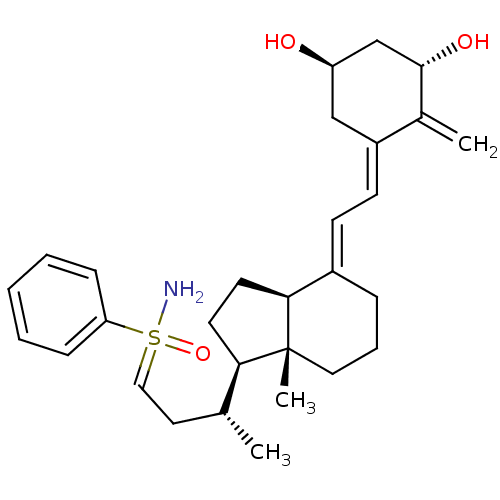
(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411322
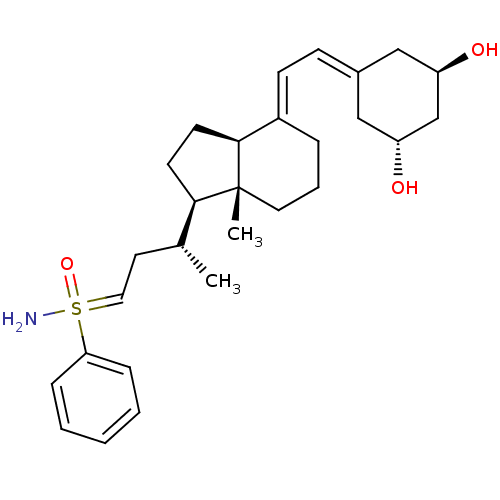
Show SMILES [#6]-[#6@H](-[#6]-[#6]=S([#7])(=O)c1ccccc1)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C28H41NO3S/c1-20(14-16-33(29,32)25-8-4-3-5-9-25)26-12-13-27-22(7-6-15-28(26,27)2)11-10-21-17-23(30)19-24(31)18-21/h3-5,8-11,16,20,23-24,26-27,30-31H,6-7,12-15,17-19H2,1-2H3,(H2,29,32)/b22-11+/t20-,23-,24-,26-,27+,28-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411322

Show SMILES [#6]-[#6@H](-[#6]-[#6]=S([#7])(=O)c1ccccc1)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C28H41NO3S/c1-20(14-16-33(29,32)25-8-4-3-5-9-25)26-12-13-27-22(7-6-15-28(26,27)2)11-10-21-17-23(30)19-24(31)18-21/h3-5,8-11,16,20,23-24,26-27,30-31H,6-7,12-15,17-19H2,1-2H3,(H2,29,32)/b22-11+/t20-,23-,24-,26-,27+,28-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200180
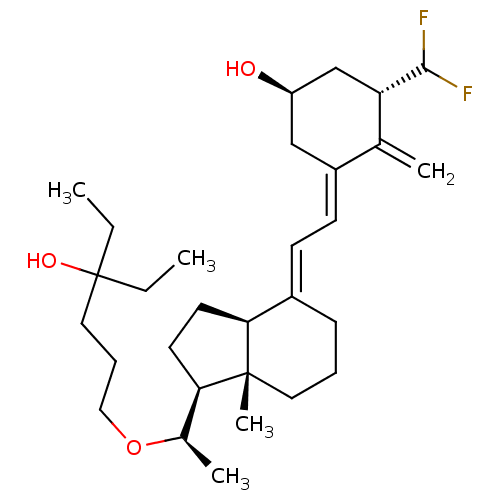
(CHEMBL376093 | KSP-BCS-1-alpha-CHF2-20-epi-22-oxab...)Show SMILES CCC(O)(CC)CCCO[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](C(F)F)C1=C |r| Show InChI InChI=1S/C30H48F2O3/c1-6-30(34,7-2)16-9-17-35-21(4)26-13-14-27-22(10-8-15-29(26,27)5)11-12-23-18-24(33)19-25(20(23)3)28(31)32/h11-12,21,24-28,33-34H,3,6-10,13-19H2,1-2,4-5H3/b22-11+,23-12-/t21-,24-,25+,26-,27+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas
Curated by ChEMBL
| Assay Description
Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assay |
J Med Chem 49: 7513-7 (2006)
Article DOI: 10.1021/jm0609925
BindingDB Entry DOI: 10.7270/Q2XG9QS7 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411326
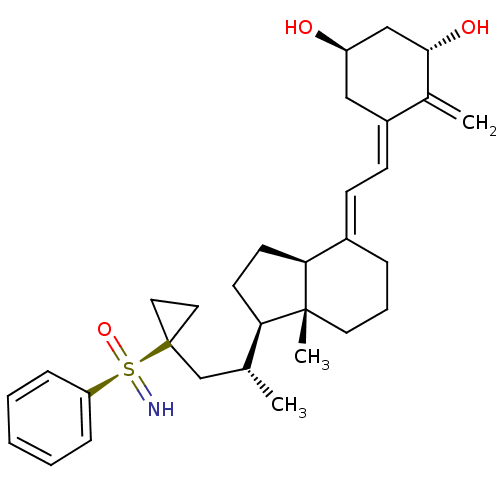
(CHEMBL2092724)Show SMILES C[C@H](CC1(CC1)[S@](=N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C31H43NO3S/c1-21(20-31(16-17-31)36(32,35)26-9-5-4-6-10-26)27-13-14-28-23(8-7-15-30(27,28)3)11-12-24-18-25(33)19-29(34)22(24)2/h4-6,9-12,21,25,27-29,32-34H,2,7-8,13-20H2,1,3H3/b23-11+,24-12-/t21-,25-,27-,28+,29+,30-,36-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925

(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411324
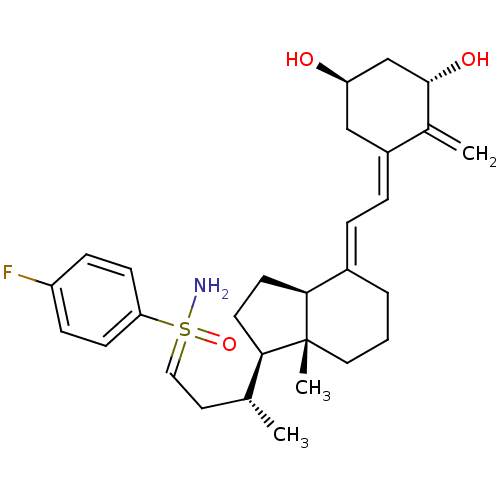
Show SMILES C[C@H](CC=S(N)(=O)c1ccc(F)cc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H40FNO3S/c1-19(14-16-35(31,34)25-10-8-23(30)9-11-25)26-12-13-27-21(5-4-15-29(26,27)3)6-7-22-17-24(32)18-28(33)20(22)2/h6-11,16,19,24,26-28,32-33H,2,4-5,12-15,17-18H2,1,3H3,(H2,31,34)/b21-6+,22-7-/t19-,24-,26-,27+,28+,29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50158486

((1R,3S,5Z)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R)-4-(benzen...)Show SMILES C[C@H](CCS(=O)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H40O4S/c1-20(15-17-34(32,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(30)19-28(31)21(23)2/h4-6,9-12,20,24,26-28,30-31H,2,7-8,13-19H2,1,3H3/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200179
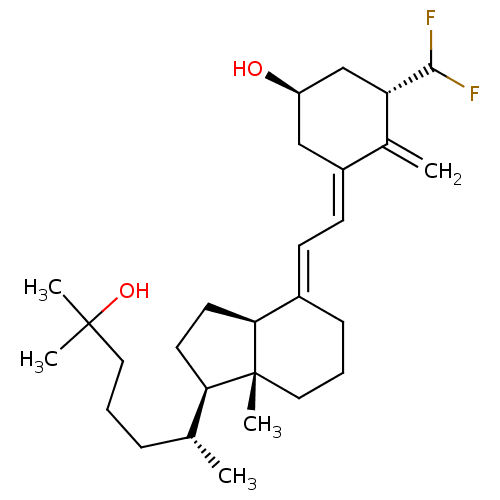
((1S,3S,Z)-3-(difluoromethyl)-5-((E)-2-((1R,3aS,7aR...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](C(F)F)C1=C |r| Show InChI InChI=1S/C28H44F2O2/c1-18(8-6-14-27(3,4)32)24-12-13-25-20(9-7-15-28(24,25)5)10-11-21-16-22(31)17-23(19(21)2)26(29)30/h10-11,18,22-26,31-32H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas
Curated by ChEMBL
| Assay Description
Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assay |
J Med Chem 49: 7513-7 (2006)
Article DOI: 10.1021/jm0609925
BindingDB Entry DOI: 10.7270/Q2XG9QS7 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411324

Show SMILES C[C@H](CC=S(N)(=O)c1ccc(F)cc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H40FNO3S/c1-19(14-16-35(31,34)25-10-8-23(30)9-11-25)26-12-13-27-21(5-4-15-29(26,27)3)6-7-22-17-24(32)18-28(33)20(22)2/h6-11,16,19,24,26-28,32-33H,2,4-5,12-15,17-18H2,1,3H3,(H2,31,34)/b21-6+,22-7-/t19-,24-,26-,27+,28+,29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50224338
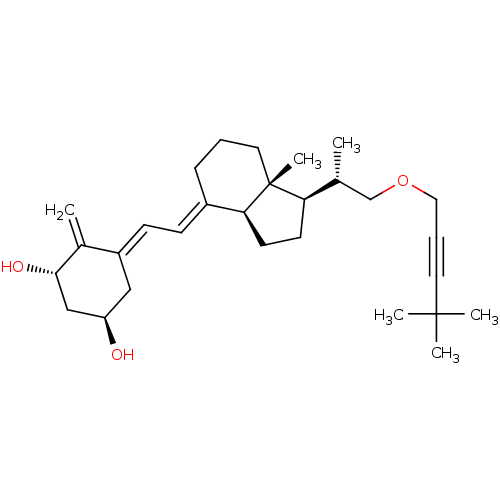
((1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-1-((S)-1-(4,4-dim...)Show SMILES C[C@H](COCC#CC(C)(C)C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C29H44O3/c1-20(19-32-16-8-14-28(3,4)5)25-12-13-26-22(9-7-15-29(25,26)6)10-11-23-17-24(30)18-27(31)21(23)2/h10-11,20,24-27,30-31H,2,7,9,12-13,15-19H2,1,3-6H3/b22-10+,23-11-/t20-,24-,25-,26+,27+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Displacement of 1alpha, 25-(OH)[3H]D3 from human VDR expressed in COS1 cells |
J Med Chem 50: 5824-32 (2007)
Article DOI: 10.1021/jm070882d
BindingDB Entry DOI: 10.7270/Q2NK3DSS |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200181
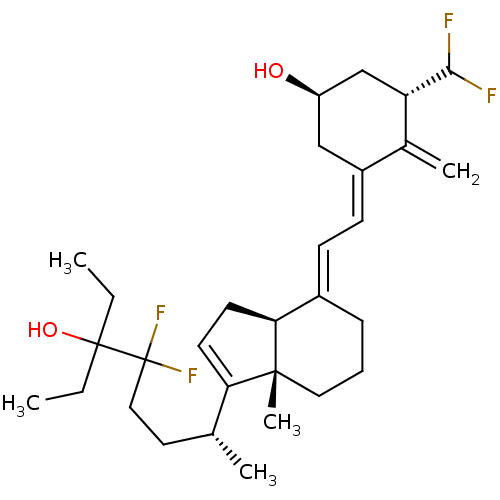
(CHEMBL220140 | KSP-BCS-1-alpha-CHF2-1624F2-2)Show SMILES CCC(O)(CC)C(F)(F)CC[C@@H](C)C1=CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](C(F)F)C1=C |r,t:13| Show InChI InChI=1S/C30H44F4O2/c1-6-29(36,7-2)30(33,34)16-14-19(3)25-12-13-26-21(9-8-15-28(25,26)5)10-11-22-17-23(35)18-24(20(22)4)27(31)32/h10-12,19,23-24,26-27,35-36H,4,6-9,13-18H2,1-3,5H3/b21-10+,22-11-/t19-,23-,24+,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas
Curated by ChEMBL
| Assay Description
Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assay |
J Med Chem 49: 7513-7 (2006)
Article DOI: 10.1021/jm0609925
BindingDB Entry DOI: 10.7270/Q2XG9QS7 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50411323
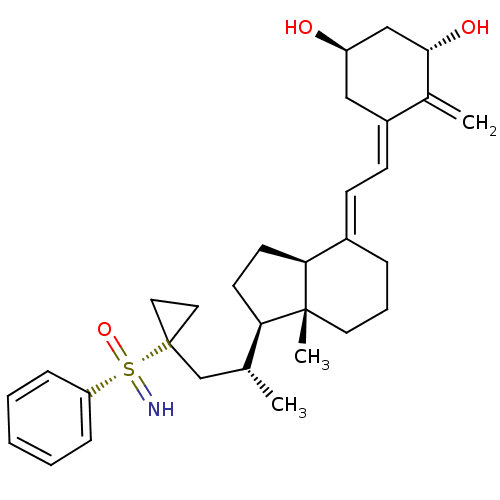
(CHEMBL2093103)Show SMILES C[C@H](CC1(CC1)[S@@](=N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C31H43NO3S/c1-21(20-31(16-17-31)36(32,35)26-9-5-4-6-10-26)27-13-14-28-23(8-7-15-30(27,28)3)11-12-24-18-25(33)19-29(34)22(24)2/h4-6,9-12,21,25,27-29,32-34H,2,7-8,13-20H2,1,3H3/b23-11+,24-12-/t21-,25-,27-,28+,29+,30-,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50224334
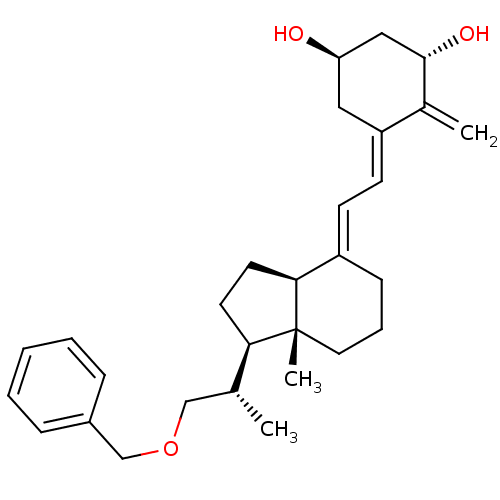
((1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-1-((S)-1-(benzylo...)Show SMILES C[C@H](COCc1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C29H40O3/c1-20(18-32-19-22-8-5-4-6-9-22)26-13-14-27-23(10-7-15-29(26,27)3)11-12-24-16-25(30)17-28(31)21(24)2/h4-6,8-9,11-12,20,25-28,30-31H,2,7,10,13-19H2,1,3H3/b23-11+,24-12-/t20-,25-,26-,27+,28+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Displacement of 1alpha, 25-(OH)[3H]D3 from human VDR expressed in COS1 cells |
J Med Chem 50: 5824-32 (2007)
Article DOI: 10.1021/jm070882d
BindingDB Entry DOI: 10.7270/Q2NK3DSS |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370926
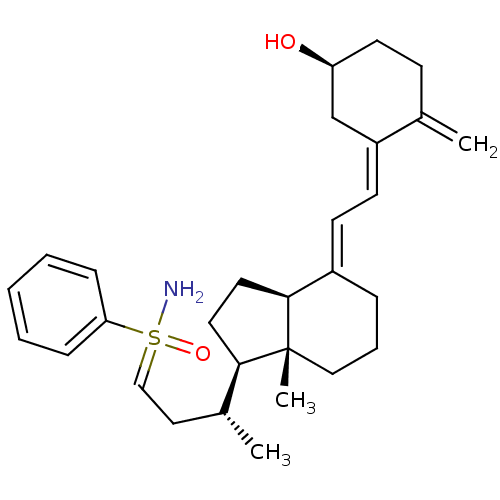
(CHEMBL1627204)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)CCC1=C |r| Show InChI InChI=1S/C29H41NO2S/c1-21-11-14-25(31)20-24(21)13-12-23-8-7-18-29(3)27(15-16-28(23)29)22(2)17-19-33(30,32)26-9-5-4-6-10-26/h4-6,9-10,12-13,19,22,25,27-28,31H,1,7-8,11,14-18,20H2,2-3H3,(H2,30,32)/b23-12+,24-13-/t22-,25+,27-,28+,29-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50224337
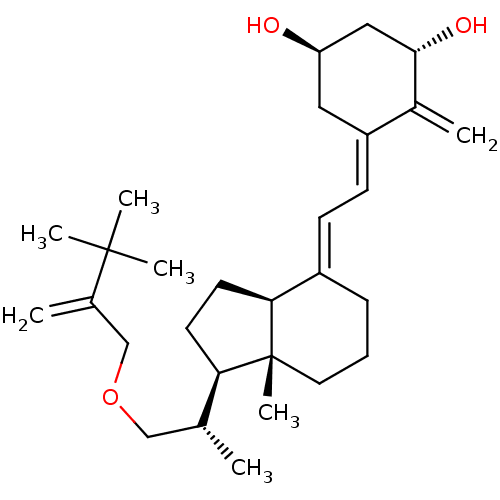
((1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-1-((S)-1-(3,3-dim...)Show SMILES C[C@H](COCC(=C)C(C)(C)C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C29H46O3/c1-19(17-32-18-20(2)28(4,5)6)25-12-13-26-22(9-8-14-29(25,26)7)10-11-23-15-24(30)16-27(31)21(23)3/h10-11,19,24-27,30-31H,2-3,8-9,12-18H2,1,4-7H3/b22-10+,23-11-/t19-,24-,25-,26+,27+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Displacement of 1alpha, 25-(OH)[3H]D3 from human VDR expressed in COS1 cells |
J Med Chem 50: 5824-32 (2007)
Article DOI: 10.1021/jm070882d
BindingDB Entry DOI: 10.7270/Q2NK3DSS |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50224332
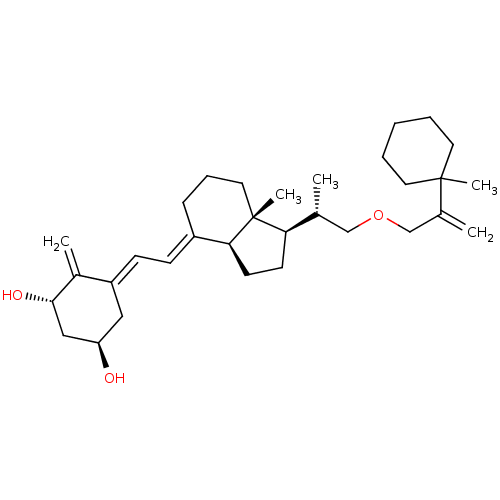
((1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-7a-methyl-1-((S)-...)Show SMILES C[C@H](COCC(=C)C1(C)CCCCC1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C32H50O3/c1-22(20-35-21-23(2)31(4)15-7-6-8-16-31)28-13-14-29-25(10-9-17-32(28,29)5)11-12-26-18-27(33)19-30(34)24(26)3/h11-12,22,27-30,33-34H,2-3,6-10,13-21H2,1,4-5H3/b25-11+,26-12-/t22-,27-,28-,29+,30+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Displacement of 1alpha, 25-(OH)[3H]D3 from human VDR expressed in COS1 cells |
J Med Chem 50: 5824-32 (2007)
Article DOI: 10.1021/jm070882d
BindingDB Entry DOI: 10.7270/Q2NK3DSS |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50224335
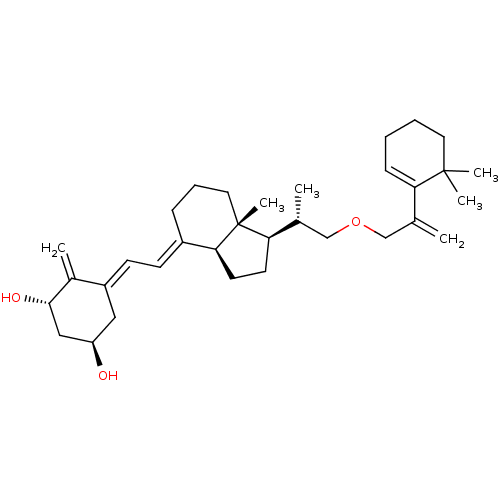
((1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-1-((S)-1-(2-(6,6-...)Show SMILES C[C@H](COCC(=C)C1=CCCCC1(C)C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |t:7| Show InChI InChI=1S/C33H50O3/c1-22(28-11-7-8-16-32(28,4)5)20-36-21-23(2)29-14-15-30-25(10-9-17-33(29,30)6)12-13-26-18-27(34)19-31(35)24(26)3/h11-13,23,27,29-31,34-35H,1,3,7-10,14-21H2,2,4-6H3/b25-12+,26-13-/t23-,27-,29-,30+,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Displacement of 1alpha, 25-(OH)[3H]D3 from human VDR expressed in COS1 cells |
J Med Chem 50: 5824-32 (2007)
Article DOI: 10.1021/jm070882d
BindingDB Entry DOI: 10.7270/Q2NK3DSS |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370927
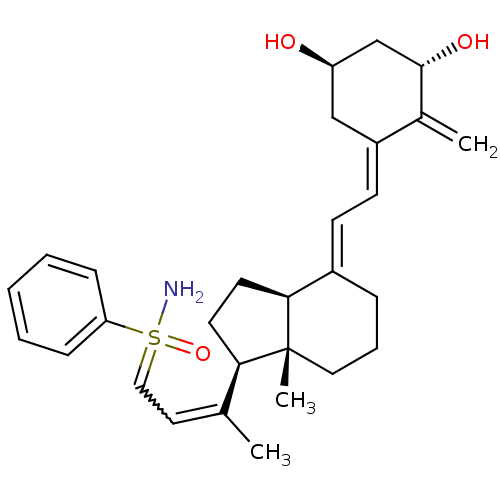
(CHEMBL1627205 | CHEMBL1627234)Show SMILES CC(=CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r,w:2.2| Show InChI InChI=1S/C29H39NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,15,17,24,26-28,31-32H,2,7-8,13-14,16,18-19H2,1,3H3,(H2,30,33)/b20-15?,22-11+,23-12-/t24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925

(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP27B hydroxylase expressed in SW900 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370932
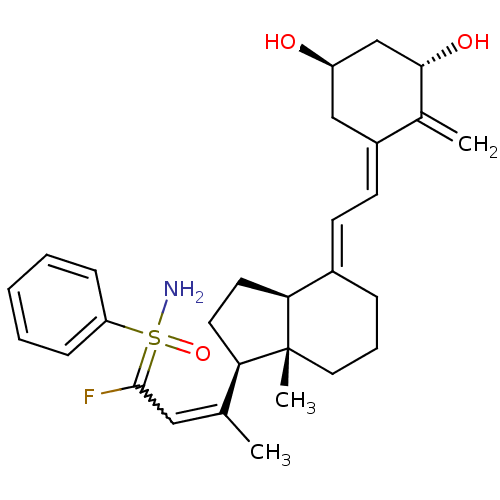
(CHEMBL1627208)Show SMILES CC(=CC(F)=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r,w:2.2| Show InChI InChI=1S/C29H38FNO3S/c1-19(16-28(30)35(31,34)24-9-5-4-6-10-24)25-13-14-26-21(8-7-15-29(25,26)3)11-12-22-17-23(32)18-27(33)20(22)2/h4-6,9-12,16,23,25-27,32-33H,2,7-8,13-15,17-18H2,1,3H3,(H2,31,34)/b19-16?,21-11+,22-12-/t23-,25-,26+,27+,29-,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
Sterol 26-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370925

(CHEMBL1627233)Show SMILES C[C@H](CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C29H41NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,17,20,24,26-28,31-32H,2,7-8,13-16,18-19H2,1,3H3,(H2,30,33)/b22-11+,23-12-/t20-,24-,26-,27+,28+,29-,34?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP27A1 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50370927

(CHEMBL1627205 | CHEMBL1627234)Show SMILES CC(=CC=S(N)(=O)c1ccccc1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r,w:2.2| Show InChI InChI=1S/C29H39NO3S/c1-20(15-17-34(30,33)25-9-5-4-6-10-25)26-13-14-27-22(8-7-16-29(26,27)3)11-12-23-18-24(31)19-28(32)21(23)2/h4-6,9-12,15,17,24,26-28,31-32H,2,7-8,13-14,16,18-19H2,1,3H3,(H2,30,33)/b20-15?,22-11+,23-12-/t24-,26-,27+,28+,29-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP24 hydroxylase expressed in V79 cells |
J Med Chem 47: 6854-63 (2004)
Article DOI: 10.1021/jm040129+
BindingDB Entry DOI: 10.7270/Q2ZW1MRZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data