

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030532 (CHEMBL62196 | Ethyl-methyl-carbamic acid 4-(2,6-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rC)-oligo (dG) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030537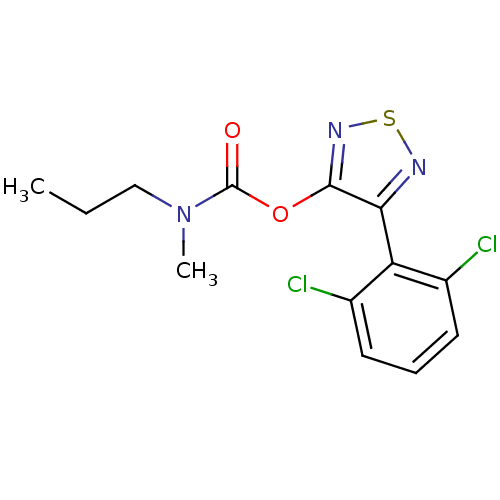 (CHEMBL60314 | Methyl-propyl-carbamic acid 4-(2,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rC)-oligo (dG) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50292354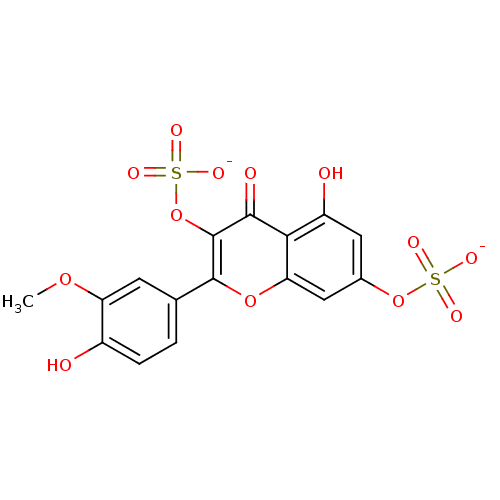 (CHEMBL463755 | Isorhamnetin 3,7-disulfate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030532 (CHEMBL62196 | Ethyl-methyl-carbamic acid 4-(2,6-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50292357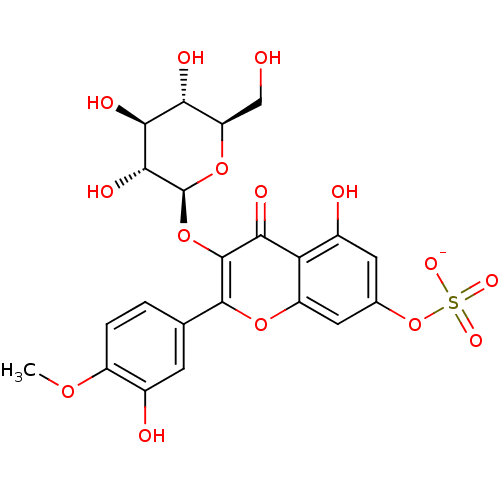 (CHEMBL458251 | Tamarixetin 3-glucoside-7-sulfate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030537 (CHEMBL60314 | Methyl-propyl-carbamic acid 4-(2,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030540 (Butyl-methyl-carbamic acid 4-(2,6-dichloro-phenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030540 (Butyl-methyl-carbamic acid 4-(2,6-dichloro-phenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50241354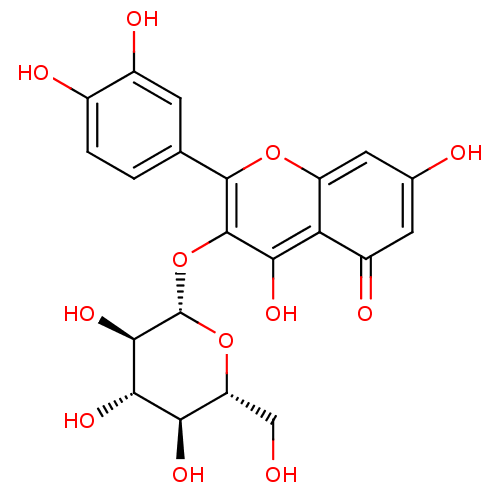 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030539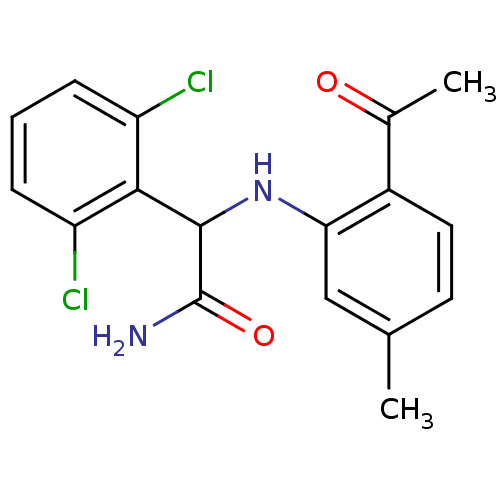 (2-(2-Acetyl-5-methyl-phenylamino)-2-(2,6-dichloro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50030539 (2-(2-Acetyl-5-methyl-phenylamino)-2-(2,6-dichloro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50292356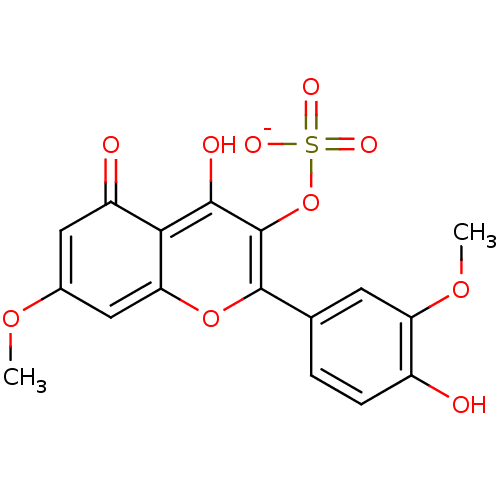 (CHEMBL457815 | Rhamnazin-3-sulfate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tosoh Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 RT activity using template primer,poly (rA)-oligo (dT) | J Med Chem 38: 2038-40 (1995) BindingDB Entry DOI: 10.7270/Q2BZ6532 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50292352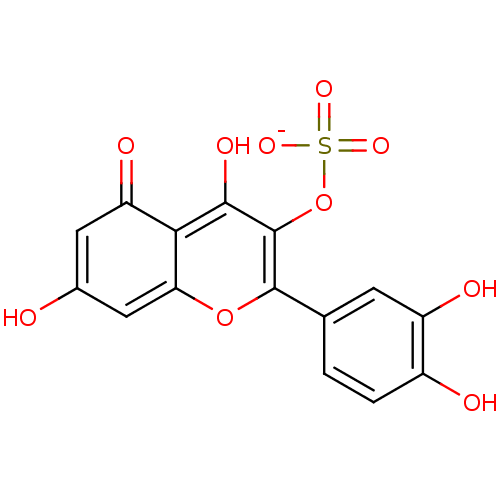 (CHEMBL463753 | potassium 2-(3,4-dihydroxyphenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50292353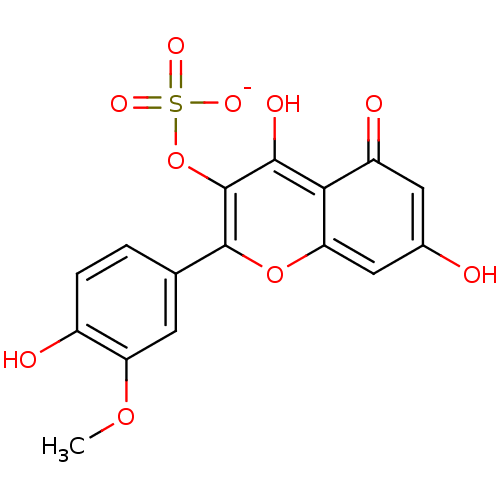 (CHEMBL471479 | Percicarin | Persicarin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM50292355 (CHEMBL457148 | Rhamnazin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Sus scrofa) | BDBM23409 (3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukuyama University Curated by ChEMBL | Assay Description Inhibition of pig lens aldose reductase by spectrophotometry | J Nat Prod 59: 443-5 (1996) Article DOI: 10.1021/np9601622 BindingDB Entry DOI: 10.7270/Q29Z94X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||