 Found 64 hits with Last Name = 'singla' and Initial = 's'
Found 64 hits with Last Name = 'singla' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50218528
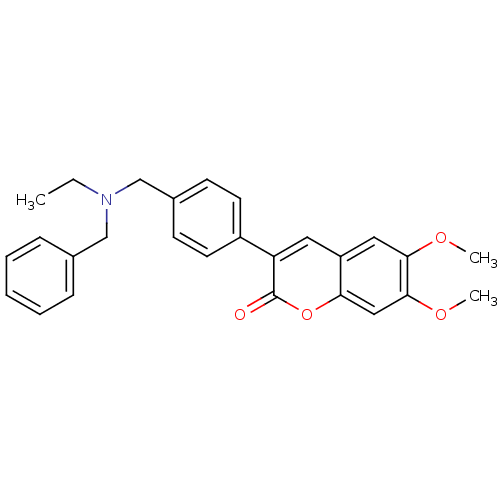
(3-(4-((benzyl(ethyl)amino)methyl)phenyl)-6,7-dimet...)Show SMILES CCN(Cc1ccccc1)Cc1ccc(cc1)-c1cc2cc(OC)c(OC)cc2oc1=O Show InChI InChI=1S/C27H27NO4/c1-4-28(17-19-8-6-5-7-9-19)18-20-10-12-21(13-11-20)23-14-22-15-25(30-2)26(31-3)16-24(22)32-27(23)29/h5-16H,4,17-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase using acetylthiocholine iodide as substrate incubated for 20 mins prior to substrate addition by Ellmans met... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10949
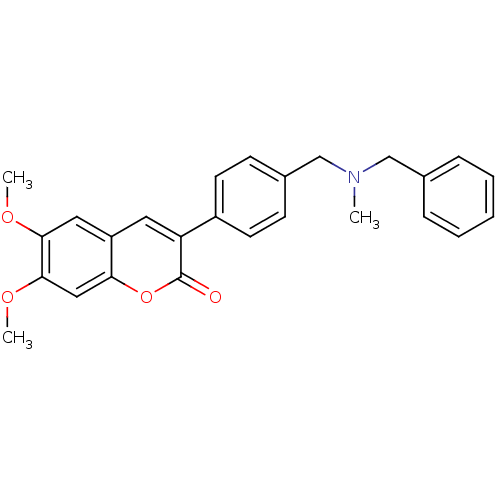
(3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccccc4)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C26H25NO4/c1-27(16-18-7-5-4-6-8-18)17-19-9-11-20(12-10-19)22-13-21-14-24(29-2)25(30-3)15-23(21)31-26(22)28/h4-15H,16-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM13066
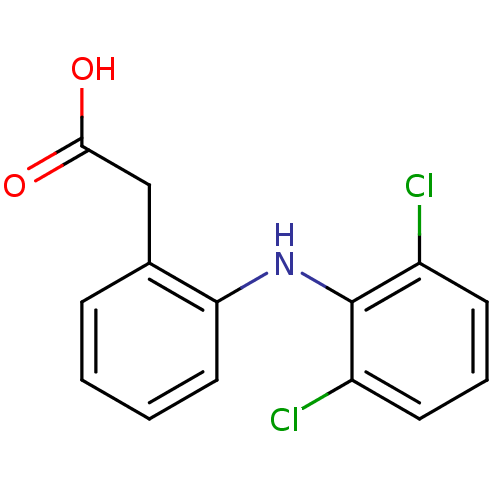
(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015563

(CHEMBL1242554)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H15N5O3/c29-28(30)19-13-11-16(12-14-19)21-20(15-27(26-21)18-9-5-2-6-10-18)23-25-24-22(31-23)17-7-3-1-4-8-17/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015564

(CHEMBL3265358)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14N6O3/c29-28(30)18-8-6-15(7-9-18)20-19(14-27(26-20)17-4-2-1-3-5-17)22-25-24-21(31-22)16-10-12-23-13-11-16/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015565
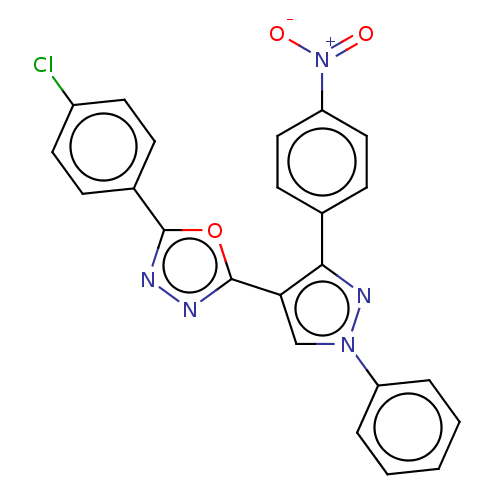
(CHEMBL3265351)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H14ClN5O3/c24-17-10-6-16(7-11-17)22-25-26-23(32-22)20-14-28(18-4-2-1-3-5-18)27-21(20)15-8-12-19(13-9-15)29(30)31/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015566

(CHEMBL1242275)Show SMILES c1c(-c2nnc(o2)-c2ccccc2)c(nn1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H16N4O/c1-4-10-17(11-5-1)21-20(16-27(26-21)19-14-8-3-9-15-19)23-25-24-22(28-23)18-12-6-2-7-13-18/h1-16H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015567

(CHEMBL3265354)Show SMILES Fc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14FN5O/c23-17-8-6-15(7-9-17)20-19(14-28(27-20)18-4-2-1-3-5-18)22-26-25-21(29-22)16-10-12-24-13-11-16/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534614

(CHEMBL4470726)Show InChI InChI=1S/C22H24N2O3/c25-22-17-21(19-9-4-5-10-20(19)27-22)26-16-6-11-23-12-14-24(15-13-23)18-7-2-1-3-8-18/h1-5,7-10,17H,6,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13066

(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015568
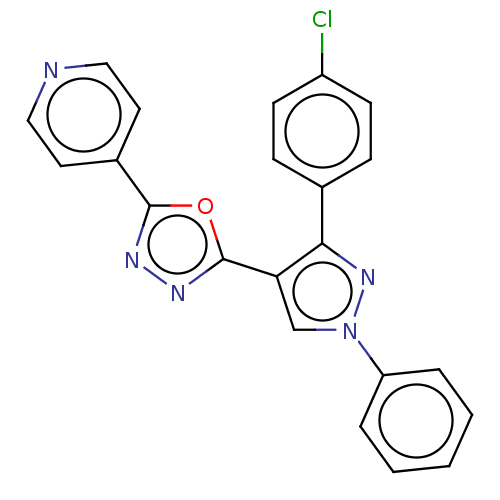
(CHEMBL3265355)Show SMILES Clc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14ClN5O/c23-17-8-6-15(7-9-17)20-19(14-28(27-20)18-4-2-1-3-5-18)22-26-25-21(29-22)16-10-12-24-13-11-16/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015569

(CHEMBL1242276)Show SMILES Clc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H15ClN4O/c24-18-13-11-16(12-14-18)21-20(15-28(27-21)19-9-5-2-6-10-19)23-26-25-22(29-23)17-7-3-1-4-8-17/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534623
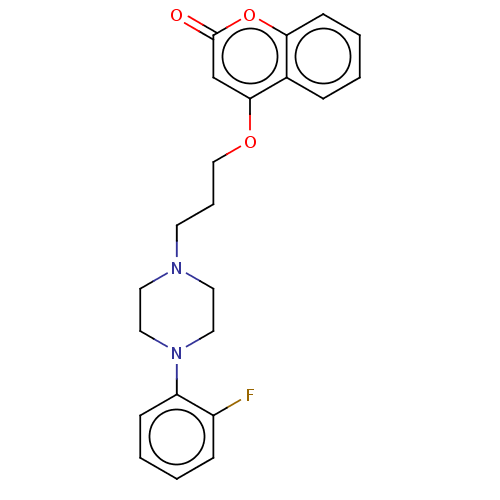
(CHEMBL4528042)Show InChI InChI=1S/C22H23FN2O3/c23-18-7-2-3-8-19(18)25-13-11-24(12-14-25)10-5-15-27-21-16-22(26)28-20-9-4-1-6-17(20)21/h1-4,6-9,16H,5,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015570
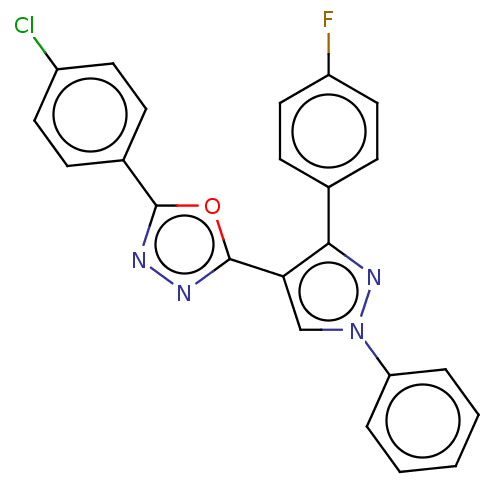
(CHEMBL3265347)Show SMILES Fc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H14ClFN4O/c24-17-10-6-16(7-11-17)22-26-27-23(30-22)20-14-29(19-4-2-1-3-5-19)28-21(20)15-8-12-18(25)13-9-15/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015571
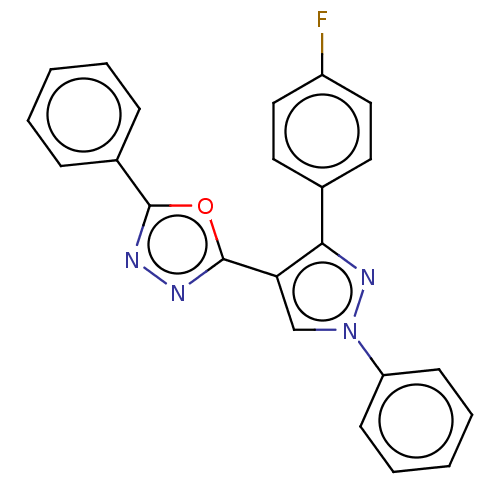
(CHEMBL1242364)Show SMILES Fc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H15FN4O/c24-18-13-11-16(12-14-18)21-20(15-28(27-21)19-9-5-2-6-10-19)23-26-25-22(29-23)17-7-3-1-4-8-17/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015572

(CHEMBL3265348)Show SMILES Clc1ccc(cc1)-c1nnc(o1)-c1cn(nc1-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H14Cl2N4O/c24-17-10-6-15(7-11-17)21-20(14-29(28-21)19-4-2-1-3-5-19)23-27-26-22(30-23)16-8-12-18(25)13-9-16/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534624

(CHEMBL4455973)Show SMILES Fc1ccc(cc1)N1CCN(CCCOc2cc(=O)oc3ccccc23)CC1 Show InChI InChI=1S/C22H23FN2O3/c23-17-6-8-18(9-7-17)25-13-11-24(12-14-25)10-3-15-27-21-16-22(26)28-20-5-2-1-4-19(20)21/h1-2,4-9,16H,3,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015573

(CHEMBL3265352)Show SMILES c1c(-c2nnc(o2)-c2ccncc2)c(nn1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C22H15N5O/c1-3-7-16(8-4-1)20-19(15-27(26-20)18-9-5-2-6-10-18)22-25-24-21(28-22)17-11-13-23-14-12-17/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534615
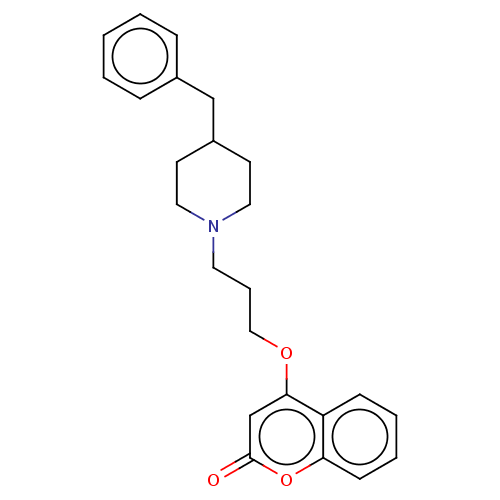
(CHEMBL4592519)Show InChI InChI=1S/C24H27NO3/c26-24-18-23(21-9-4-5-10-22(21)28-24)27-16-6-13-25-14-11-20(12-15-25)17-19-7-2-1-3-8-19/h1-5,7-10,18,20H,6,11-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534621
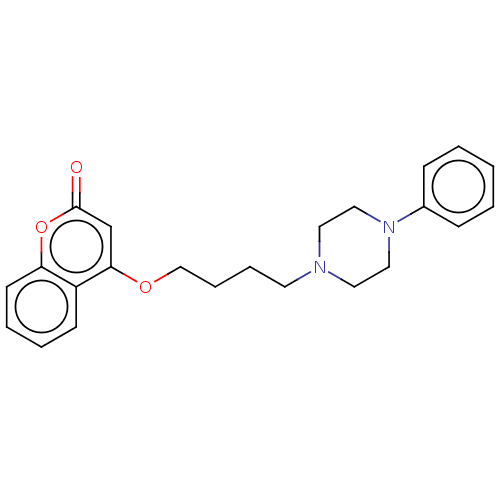
(CHEMBL4550487)Show InChI InChI=1S/C23H26N2O3/c26-23-18-22(20-10-4-5-11-21(20)28-23)27-17-7-6-12-24-13-15-25(16-14-24)19-8-2-1-3-9-19/h1-5,8-11,18H,6-7,12-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534620

(CHEMBL4535870)Show InChI InChI=1S/C21H23N3O3/c25-21-16-19(17-6-1-2-7-18(17)27-21)26-15-5-10-23-11-13-24(14-12-23)20-8-3-4-9-22-20/h1-4,6-9,16H,5,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534612
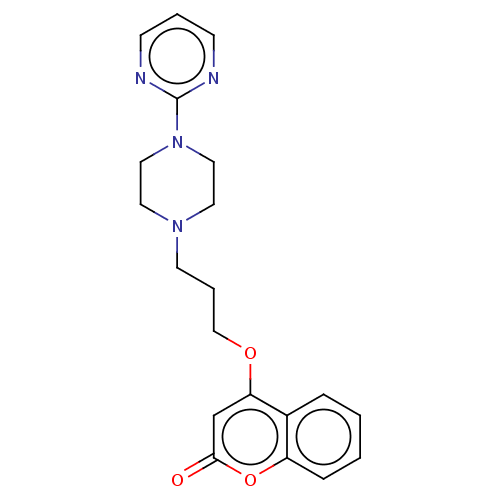
(CHEMBL4544371)Show InChI InChI=1S/C20H22N4O3/c25-19-15-18(16-5-1-2-6-17(16)27-19)26-14-4-9-23-10-12-24(13-11-23)20-21-7-3-8-22-20/h1-3,5-8,15H,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015574
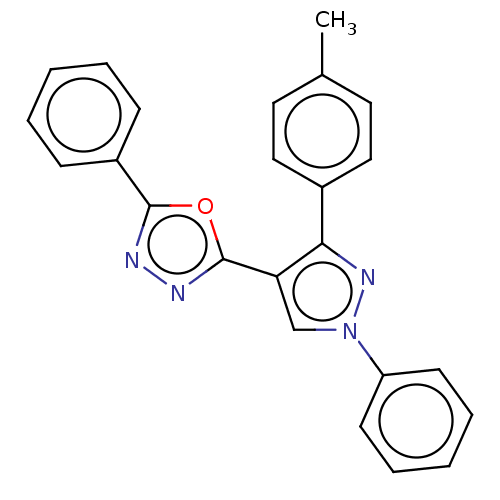
(CHEMBL1242459)Show SMILES Cc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C24H18N4O/c1-17-12-14-18(15-13-17)22-21(16-28(27-22)20-10-6-3-7-11-20)24-26-25-23(29-24)19-8-4-2-5-9-19/h2-16H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534618

(CHEMBL4461381)Show InChI InChI=1S/C23H25FN2O3/c24-19-8-2-3-9-20(19)26-14-12-25(13-15-26)11-5-6-16-28-22-17-23(27)29-21-10-4-1-7-18(21)22/h1-4,7-10,17H,5-6,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534626
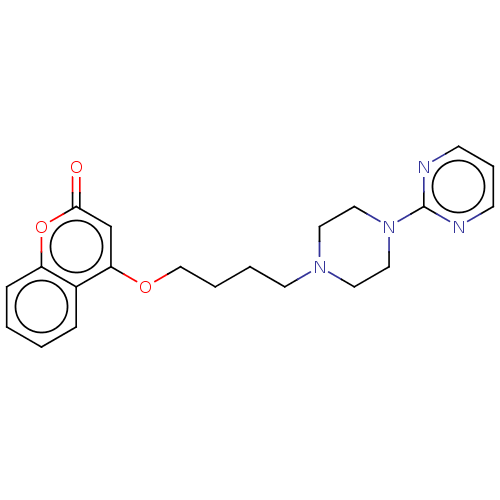
(CHEMBL4450711)Show InChI InChI=1S/C21H24N4O3/c26-20-16-19(17-6-1-2-7-18(17)28-20)27-15-4-3-10-24-11-13-25(14-12-24)21-22-8-5-9-23-21/h1-2,5-9,16H,3-4,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534619

(CHEMBL4458007)Show InChI InChI=1S/C22H25N3O3/c26-22-17-20(18-7-1-2-8-19(18)28-22)27-16-6-5-11-24-12-14-25(15-13-24)21-9-3-4-10-23-21/h1-4,7-10,17H,5-6,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015575
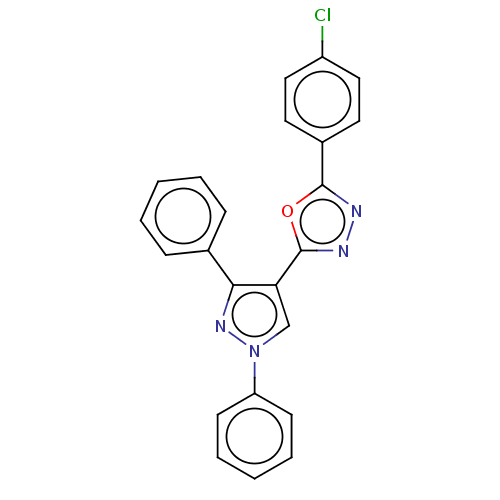
(CHEMBL3265345)Show SMILES Clc1ccc(cc1)-c1nnc(o1)-c1cn(nc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H15ClN4O/c24-18-13-11-17(12-14-18)22-25-26-23(29-22)20-15-28(19-9-5-2-6-10-19)27-21(20)16-7-3-1-4-8-16/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015576

(CHEMBL3265356)Show SMILES Brc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14BrN5O/c23-17-8-6-15(7-9-17)20-19(14-28(27-20)18-4-2-1-3-5-18)22-26-25-21(29-22)16-10-12-24-13-11-16/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015577
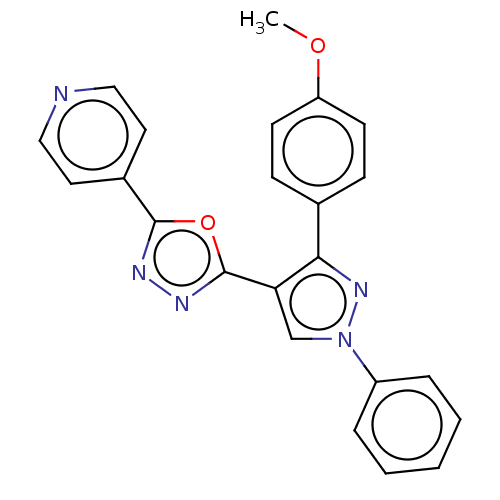
(CHEMBL3265357)Show SMILES COc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C23H17N5O2/c1-29-19-9-7-16(8-10-19)21-20(15-28(27-21)18-5-3-2-4-6-18)23-26-25-22(30-23)17-11-13-24-14-12-17/h2-15H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534617
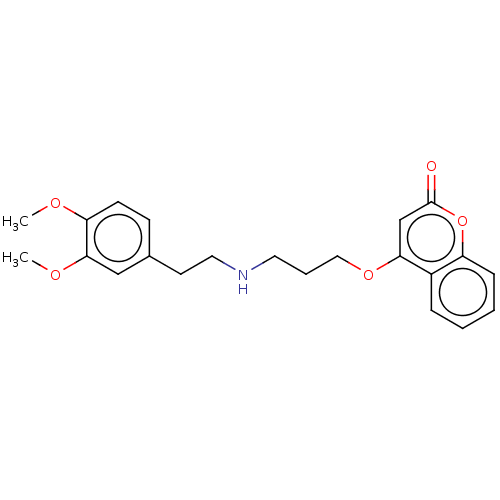
(CHEMBL4458241)Show InChI InChI=1S/C22H25NO5/c1-25-19-9-8-16(14-21(19)26-2)10-12-23-11-5-13-27-20-15-22(24)28-18-7-4-3-6-17(18)20/h3-4,6-9,14-15,23H,5,10-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015578

(CHEMBL3265349)Show SMILES Clc1ccc(cc1)-c1nnc(o1)-c1cn(nc1-c1ccc(Br)cc1)-c1ccccc1 Show InChI InChI=1S/C23H14BrClN4O/c24-17-10-6-15(7-11-17)21-20(14-29(28-21)19-4-2-1-3-5-19)23-27-26-22(30-23)16-8-12-18(25)13-9-16/h1-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015579

(CHEMBL3265353)Show SMILES Cc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C23H17N5O/c1-16-7-9-17(10-8-16)21-20(15-28(27-21)19-5-3-2-4-6-19)23-26-25-22(29-23)18-11-13-24-14-12-18/h2-15H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015580

(CHEMBL3265346)Show SMILES Cc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C24H17ClN4O/c1-16-7-9-17(10-8-16)22-21(15-29(28-22)20-5-3-2-4-6-20)24-27-26-23(30-24)18-11-13-19(25)14-12-18/h2-15H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534616

(CHEMBL4526265)Show InChI InChI=1S/C20H21NO3/c22-20-15-19(17-9-4-5-10-18(17)24-20)23-14-6-12-21-13-11-16-7-2-1-3-8-16/h1-5,7-10,15,21H,6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015581

(CHEMBL3265350)Show SMILES COc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C24H17ClN4O2/c1-30-20-13-9-16(10-14-20)22-21(15-29(28-22)19-5-3-2-4-6-19)24-27-26-23(31-24)17-7-11-18(25)12-8-17/h2-15H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534613
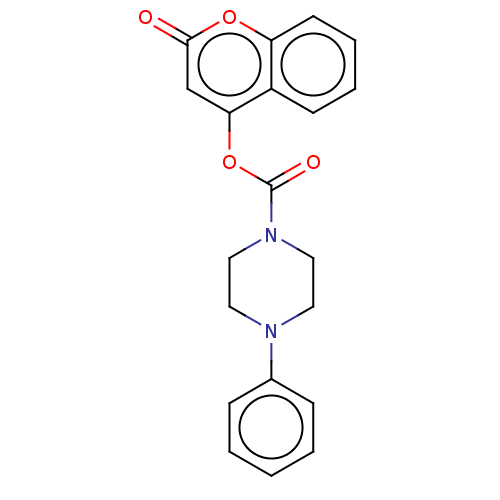
(CHEMBL4568961)Show InChI InChI=1S/C20H18N2O4/c23-19-14-18(16-8-4-5-9-17(16)25-19)26-20(24)22-12-10-21(11-13-22)15-6-2-1-3-7-15/h1-9,14H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534622

(CHEMBL4559958)Show InChI InChI=1S/C18H15NO4/c20-17-12-16(14-8-4-5-9-15(14)22-17)23-18(21)19-11-10-13-6-2-1-3-7-13/h1-9,12H,10-11H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50534625

(CHEMBL4547392)Show InChI InChI=1S/C19H26N2O4/c1-20(8-9-21-10-13-23-14-11-21)7-4-12-24-18-15-19(22)25-17-6-3-2-5-16(17)18/h2-3,5-6,15H,4,7-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in Albino LACA mouse brain homogenates using acetylthiocholine iodide as substrate measured for 2 mins by Ellman's... |
Bioorg Med Chem 24: 4587-4599 (2016)
Article DOI: 10.1016/j.bmc.2016.07.061
BindingDB Entry DOI: 10.7270/Q2765JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015582

(CHEMBL1242365)Show SMILES Brc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H15BrN4O/c24-18-13-11-16(12-14-18)21-20(15-28(27-21)19-9-5-2-6-10-19)23-26-25-22(29-23)17-7-3-1-4-8-17/h1-15H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015583

(CHEMBL1242460)Show SMILES COc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C24H18N4O2/c1-29-20-14-12-17(13-15-20)22-21(16-28(27-22)19-10-6-3-7-11-19)24-26-25-23(30-24)18-8-4-2-5-9-18/h2-16H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50015582

(CHEMBL1242365)Show SMILES Brc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H15BrN4O/c24-18-13-11-16(12-14-18)21-20(15-28(27-21)19-9-5-2-6-10-19)23-26-25-22(29-23)17-7-3-1-4-8-17/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015564

(CHEMBL3265358)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14N6O3/c29-28(30)18-8-6-15(7-9-18)20-19(14-27(26-20)17-4-2-1-3-5-17)22-25-24-21(31-22)16-10-12-23-13-11-16/h1-14H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015577

(CHEMBL3265357)Show SMILES COc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C23H17N5O2/c1-29-19-9-7-16(8-10-19)21-20(15-28(27-21)18-5-3-2-4-6-18)23-26-25-22(30-23)17-11-13-24-14-12-17/h2-15H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015576

(CHEMBL3265356)Show SMILES Brc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14BrN5O/c23-17-8-6-15(7-9-17)20-19(14-28(27-20)18-4-2-1-3-5-18)22-26-25-21(29-22)16-10-12-24-13-11-16/h1-14H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015568

(CHEMBL3265355)Show SMILES Clc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14ClN5O/c23-17-8-6-15(7-9-17)20-19(14-28(27-20)18-4-2-1-3-5-18)22-26-25-21(29-22)16-10-12-24-13-11-16/h1-14H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015567

(CHEMBL3265354)Show SMILES Fc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H14FN5O/c23-17-8-6-15(7-9-17)20-19(14-28(27-20)18-4-2-1-3-5-18)22-26-25-21(29-22)16-10-12-24-13-11-16/h1-14H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50015579

(CHEMBL3265353)Show SMILES Cc1ccc(cc1)-c1nn(cc1-c1nnc(o1)-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C23H17N5O/c1-16-7-9-17(10-8-16)21-20(15-28(27-21)19-5-3-2-4-6-19)23-26-25-22(29-23)18-11-13-24-14-12-18/h2-15H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shoolini University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 2 mins by EIA |
Eur J Med Chem 80: 167-74 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.045
BindingDB Entry DOI: 10.7270/Q2T43VNF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data