 Found 102 hits with Last Name = 'vajda' and Initial = 's'
Found 102 hits with Last Name = 'vajda' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50270877
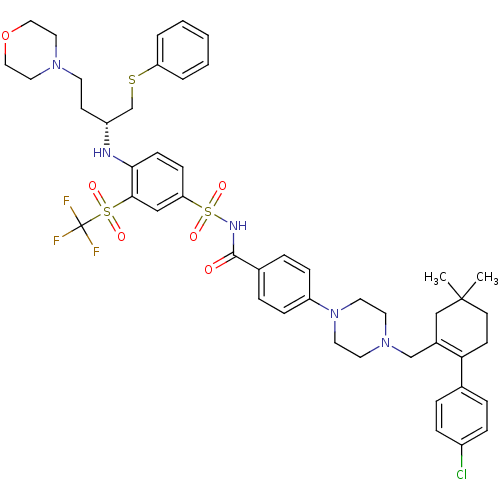
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50075098

(CHEMBL3414626 | US10143704, Compound A2 | US944606...)Show SMILES CC(C)N(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@H](CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1 |r,wU:24.27,22.24,10.11,8.8,wD:5.4,7.12,(-.55,4.43,;-1.02,5.57,;-2.24,5.73,;-.08,6.8,;1.45,6.59,;2.04,5.17,;1.23,3.86,;2.24,2.7,;3.65,3.27,;4.7,2.61,;3.54,4.8,;4.48,5.6,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.56,;-1.03,-2.79,;-2.38,-.77,;-2.38,.77,;-1.03,1.56,;.3,.77,;-.67,8.22,;-.05,9.59,;-1.48,10.19,;-2.06,11.61,;-1.11,12.83,;-1.7,14.26,;-.89,15.53,;-1.87,16.71,;-1.63,18.24,;-2.86,19.2,;-4.29,18.63,;-4.52,17.09,;-3.29,16.14,;-3.19,14.61,;-2.65,20.73,;-1.51,21.19,;-3.62,21.48,;-2.48,21.95,;-2.07,8.77,)| Show InChI InChI=1S/C30H42N8O3/c1-16(2)37(13-22-25(39)26(40)29(41-22)38-15-34-24-27(31)32-14-33-28(24)38)19-10-17(11-19)6-9-23-35-20-8-7-18(30(3,4)5)12-21(20)36-23/h7-8,12,14-17,19,22,25-26,29,39-40H,6,9-11,13H2,1-5H3,(H,35,36)(H2,31,32,33)/t17-,19+,22-,25-,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of human DOT1-like Histone H3 Methyltransferase preincubated for 30 mins followed by 3H-SAM addition and measured after 120 mins |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50148733
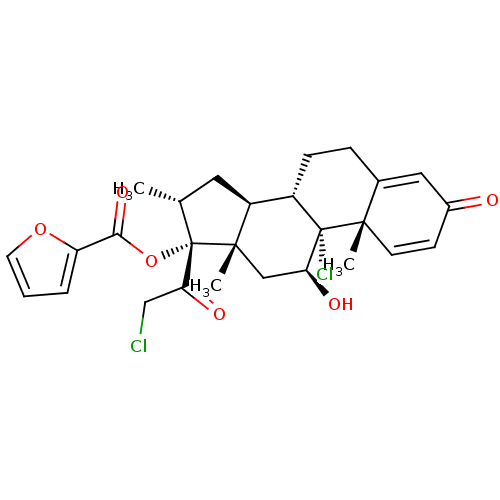
((9beta,10alpha,11alpha,14beta,16alpha,17alpha)-9,2...)Show SMILES C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(Cl)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)c1ccco1)C(=O)CCl |c:11,t:7| Show InChI InChI=1S/C27H30Cl2O6/c1-15-11-19-18-7-6-16-12-17(30)8-9-24(16,2)26(18,29)21(31)13-25(19,3)27(15,22(32)14-28)35-23(33)20-5-4-10-34-20/h4-5,8-10,12,15,18-19,21,31H,6-7,11,13-14H2,1-3H3/t15-,18+,19+,21+,24+,25+,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM15244
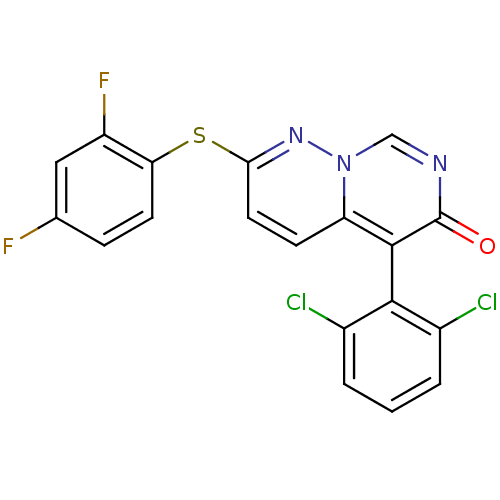
(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of P38 mitogen activated protein kinase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50500145

(CHEMBL1235987)Show SMILES NC(=O)[C@@H](CS)NC(=O)CCCCCNC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C32H41F4N5O13P2S/c33-31(34,55(49,50)51)20-9-5-18(6-10-20)14-22(29(47)38-13-3-1-2-4-25(42)40-24(17-57)28(37)46)41-30(48)23(16-27(44)45)39-26(43)15-19-7-11-21(12-8-19)32(35,36)56(52,53)54/h5-12,22-24,57H,1-4,13-17H2,(H2,37,46)(H,38,47)(H,39,43)(H,40,42)(H,41,48)(H,44,45)(H2,49,50,51)(H2,52,53,54)/t22-,23+,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50505290
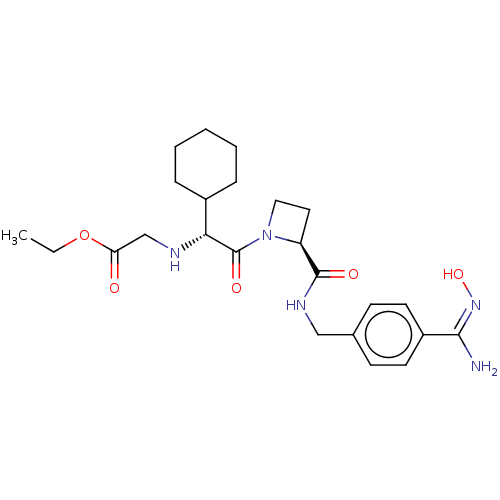
(CHEBI:65172 | Exanta | Exanta (proposed) | H 376/9...)Show SMILES CCOC(=O)CN[C@H](C1CCCCC1)C(=O)N1CC[C@H]1C(=O)NCc1ccc(cc1)C(\N)=N/O |r| Show InChI InChI=1S/C24H35N5O5/c1-2-34-20(30)15-26-21(17-6-4-3-5-7-17)24(32)29-13-12-19(29)23(31)27-14-16-8-10-18(11-9-16)22(25)28-33/h8-11,17,19,21,26,33H,2-7,12-15H2,1H3,(H2,25,28)(H,27,31)/t19-,21+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50382559
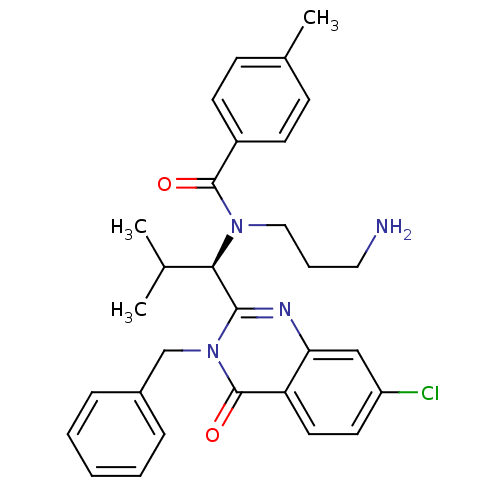
(ISPINESIB)Show SMILES CC(C)[C@@H](N(CCCN)C(=O)c1ccc(C)cc1)c1nc2cc(Cl)ccc2c(=O)n1Cc1ccccc1 Show InChI InChI=1S/C30H33ClN4O2/c1-20(2)27(34(17-7-16-32)29(36)23-12-10-21(3)11-13-23)28-33-26-18-24(31)14-15-25(26)30(37)35(28)19-22-8-5-4-6-9-22/h4-6,8-15,18,20,27H,7,16-17,19,32H2,1-3H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of Kinesin Eg5 (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50219566
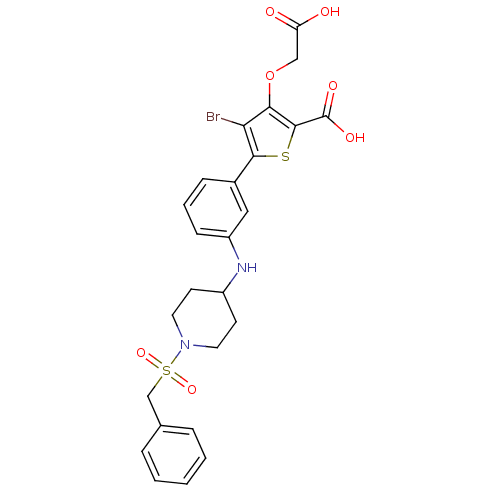
(4-bromo-3-carboxymethoxy-5-[3-(1-phenylmethanesulf...)Show SMILES OC(=O)COc1c(Br)c(sc1C(O)=O)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN2O7S2/c26-21-22(35-14-20(29)30)24(25(31)32)36-23(21)17-7-4-8-19(13-17)27-18-9-11-28(12-10-18)37(33,34)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50073316

(CHEMBL114715 | INOGATRAN | {(R)-1-Cyclohexylmethyl...)Show SMILES NC(=N)NCCCNC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C21H38N6O4/c22-21(23)25-11-6-10-24-19(30)17-9-4-5-12-27(17)20(31)16(26-14-18(28)29)13-15-7-2-1-3-8-15/h15-17,26H,1-14H2,(H,24,30)(H,28,29)(H4,22,23,25)/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM152
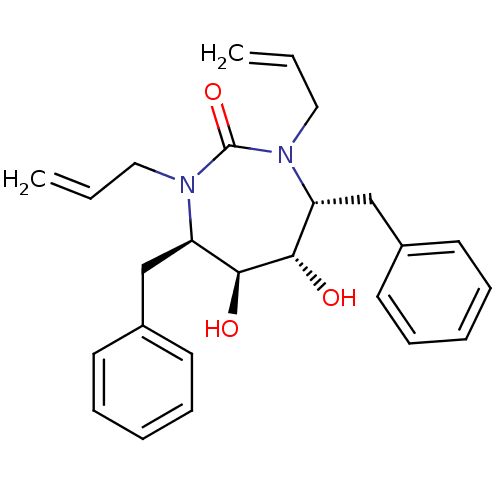
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(p...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CC=C)C(=O)N(CC=C)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C25H30N2O3/c1-3-15-26-21(17-19-11-7-5-8-12-19)23(28)24(29)22(27(16-4-2)25(26)30)18-20-13-9-6-10-14-20/h3-14,21-24,28-29H,1-2,15-18H2/t21-,22-,23+,24+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 Aspartic Protease |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50260093
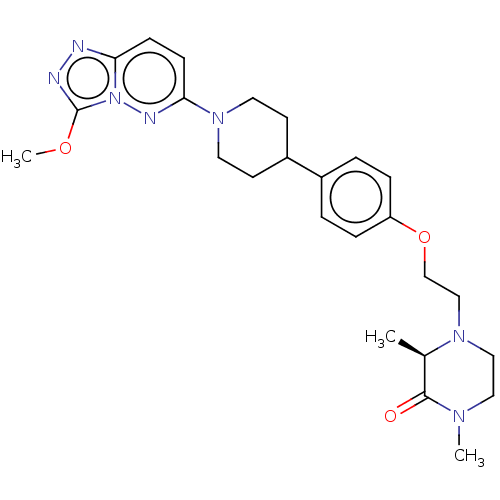
(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of BRD4 (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM22164
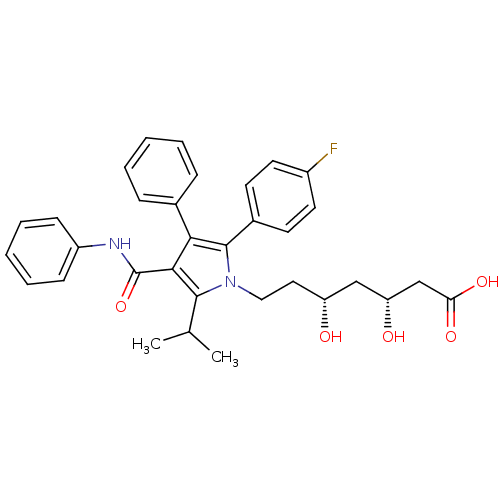
((3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylca...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H](O)C[C@@H](O)CC(O)=O)-c1ccccc1 |r| Show InChI InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA Reductase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM81734
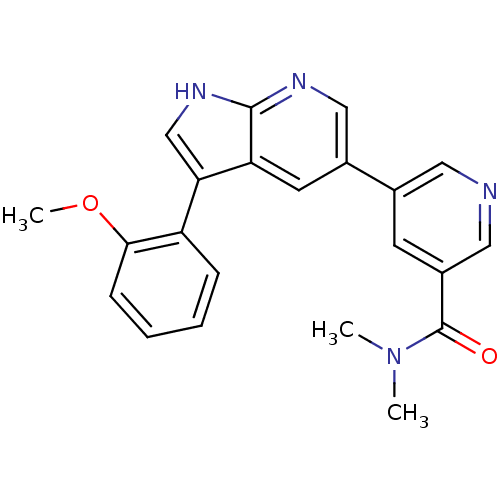
(PPY-A)Show SMILES COc1ccccc1-c1c[nH]c2ncc(cc12)-c1cncc(c1)C(=O)N(C)C Show InChI InChI=1S/C22H20N4O2/c1-26(2)22(27)16-8-14(10-23-11-16)15-9-18-19(13-25-21(18)24-12-15)17-6-4-5-7-20(17)28-3/h4-13H,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610
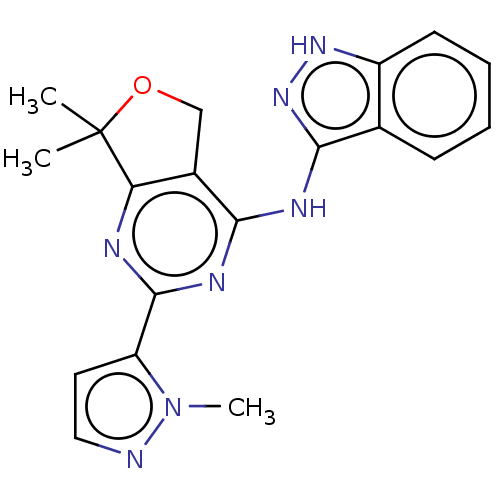
(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50505286
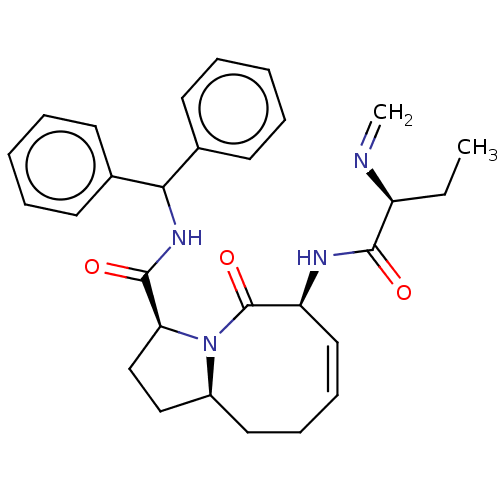
(CHEMBL1231341)Show SMILES [H][C@@](CC)(N=C)C(=O)N[C@@]1([H])\C=C/CC[C@@]2([H])CC[C@]([H])(N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1 |r,c:11| Show InChI InChI=1S/C29H34N4O3/c1-3-23(30-2)27(34)31-24-17-11-10-16-22-18-19-25(33(22)29(24)36)28(35)32-26(20-12-6-4-7-13-20)21-14-8-5-9-15-21/h4-9,11-15,17,22-26H,2-3,10,16,18-19H2,1H3,(H,31,34)(H,32,35)/b17-11-/t22-,23-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of E3 ubiquitin-protein ligase XIAP (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50526692
(CHEMBL1230881)Show SMILES CC(C)(C)c1ccc(cc1[N+]([O-])=O)C(=O)NNC(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O4/c1-22(2,3)17-12-11-15(13-19(17)26(29)30)20(27)24-25-21(28)23-18-10-6-8-14-7-4-5-9-16(14)18/h4-13H,1-3H3,(H,24,27)(H2,23,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human CHK1 (1 to 289 residues) expressed in baculovirus infected Sf21 insect cells preincubated for 15 mins followed by ATP a... |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of DOT1-like Histone H3 Methyltransferase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM19429
(4-[3-(4-fluorophenyl)-1H-pyrazol-4-yl]pyridine | S...)Show InChI InChI=1S/C14H10FN3/c15-12-3-1-11(2-4-12)14-13(9-17-18-14)10-5-7-16-8-6-10/h1-9H,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of P38 mitogen activated protein kinase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50436850
(CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...)Show SMILES CC(C)Oc1cc(C2CCNCC2)c(C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C28H36ClN5O3S/c1-17(2)37-25-15-21(20-10-12-30-13-11-20)19(5)14-24(25)33-28-31-16-22(29)27(34-28)32-23-8-6-7-9-26(23)38(35,36)18(3)4/h6-9,14-18,20,30H,10-13H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM28453
((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-[(dimeth...)Show SMILES CN(C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H18N6O3/c1-17(2)3-6-8(19)9(20)12(21-6)18-5-16-7-10(13)14-4-15-11(7)18/h4-6,8-9,12,19-20H,3H2,1-2H3,(H2,13,14,15)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of DOT1-like Histone H3 Methyltransferase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM139995
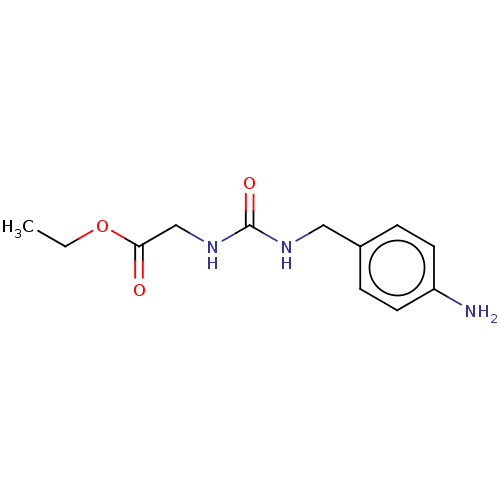
(US8901295, F428)Show InChI InChI=1S/C12H17N3O3/c1-2-18-11(16)8-15-12(17)14-7-9-3-5-10(13)6-4-9/h3-6H,2,7-8,13H2,1H3,(H2,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of Cyclophilin A (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucose-1-phosphate cytidylyltransferase
(Salmonella typhi) | BDBM50454133

(CHEBI:17677 | Cystetine Triphosphate)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H16N3O14P3/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(24-8)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,21,22)(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Binding affinity to Salmonella typhi glucose-1-phosphate cytidylyl-transferase assessed as dissociation constant by spectrophotometry |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Toll-like receptor 4
(Homo sapiens (Human)) | BDBM50274760
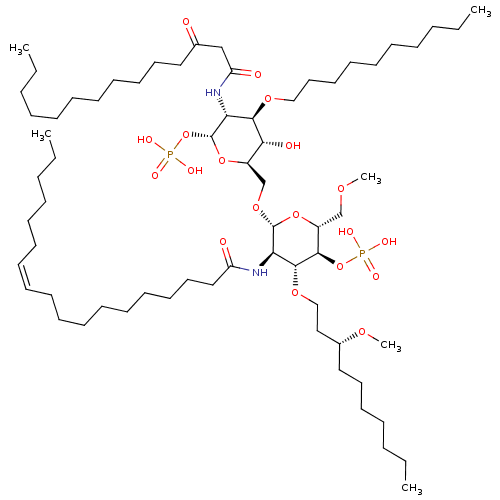
(3-O-DECYL-2-DEOXY-6-O-{2-DEOXY-3-O-[(3R)-3-METHOXY...)Show SMILES CCCCCCCCCCCC(=O)CC(=O)N[C@H]1[C@@H](OP(O)(O)=O)O[C@H](CO[C@@H]2O[C@H](COC)[C@@H](OP(O)(O)=O)[C@H](OCC[C@@H](CCCCCCC)OC)[C@H]2NC(=O)CCCCCCCCC\C=C/CCCCCC)[C@@H](O)[C@@H]1OCCCCCCCCCC |r| Show InChI InChI=1S/C66H126N2O19P2/c1-7-11-15-19-22-25-26-27-28-29-30-32-34-38-42-46-57(70)67-60-64(82-49-47-54(80-6)45-41-36-18-14-10-4)62(86-88(73,74)75)56(51-79-5)85-65(60)83-52-55-61(72)63(81-48-43-39-35-24-21-17-13-9-3)59(66(84-55)87-89(76,77)78)68-58(71)50-53(69)44-40-37-33-31-23-20-16-12-8-2/h25-26,54-56,59-66,72H,7-24,27-52H2,1-6H3,(H,67,70)(H,68,71)(H2,73,74,75)(H2,76,77,78)/b26-25-/t54-,55-,56-,59-,60-,61-,62-,63-,64-,65-,66-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Antagonist activity at TLR4 in human serum assessed as reduction in LPS-induced TNFalpha production after 3 hrs by ELISA |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453891
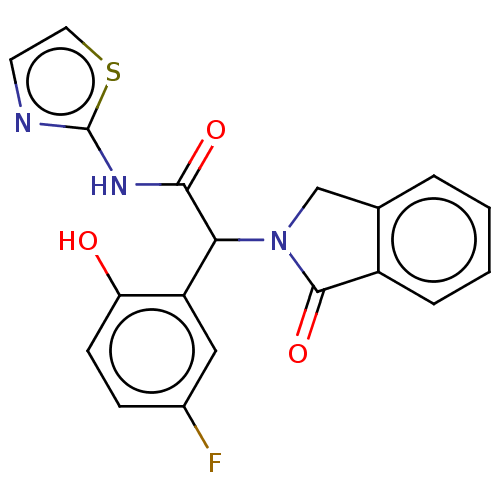
(CHEMBL4214567)Show SMILES Oc1ccc(F)cc1C(N1Cc2ccccc2C1=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H14FN3O3S/c20-12-5-6-15(24)14(9-12)16(17(25)22-19-21-7-8-27-19)23-10-11-3-1-2-4-13(11)18(23)26/h1-9,16,24H,10H2,(H,21,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR L858R/T790M mutant (696 to 1022 residues) expressed in Sf9 insect cells by HTRF assay |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50505289
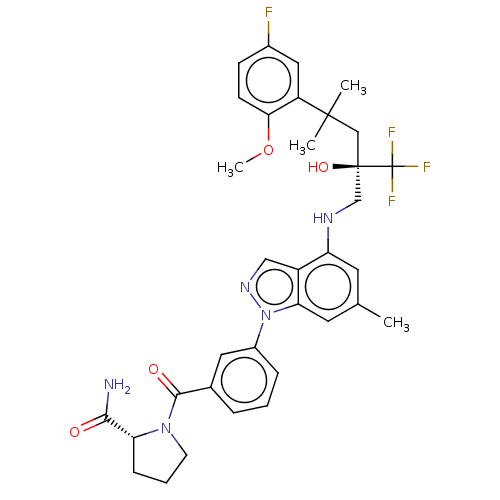
(CHEMBL1233786)Show SMILES COc1ccc(F)cc1C(C)(C)C[C@@](O)(CNc1cc(C)cc2n(ncc12)-c1cccc(c1)C(=O)N1CCC[C@@H]1C(N)=O)C(F)(F)F |r| Show InChI InChI=1S/C34H37F4N5O4/c1-20-13-26(40-19-33(46,34(36,37)38)18-32(2,3)25-16-22(35)10-11-29(25)47-4)24-17-41-43(28(24)14-20)23-8-5-7-21(15-23)31(45)42-12-6-9-27(42)30(39)44/h5,7-8,10-11,13-17,27,40,46H,6,9,12,18-19H2,1-4H3,(H2,39,44)/t27-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of glucocorticoid receptor (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391803

(CHEMBL2146888)Show SMILES NCC1(O)CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C17H15F3IN3O2/c18-11-3-2-10(16(25)24-7-17(26,6-22)8-24)15(14(11)20)23-13-4-1-9(21)5-12(13)19/h1-5,23,26H,6-8,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50500147
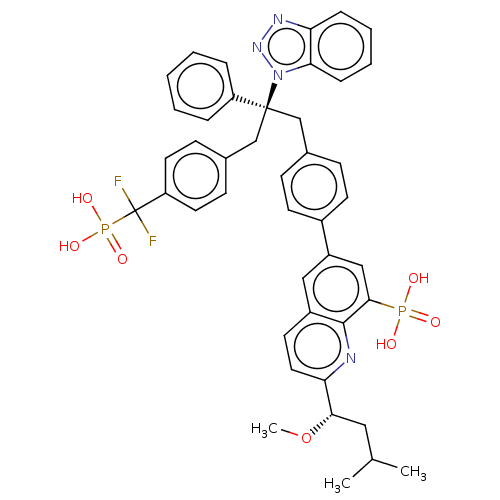
(CHEMBL3746639)Show SMILES CO[C@@H](CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(C[C@](Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 |r| Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55)/t39-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50359361

(CHEMBL1929215)Show SMILES CC(C)(C)c1cc(C(=O)N2CCNC(=O)CC2)c(NC(=O)Nc2cccc3ccccc23)s1 Show InChI InChI=1S/C25H28N4O3S/c1-25(2,3)20-15-18(23(31)29-13-11-21(30)26-12-14-29)22(33-20)28-24(32)27-19-10-6-8-16-7-4-5-9-17(16)19/h4-10,15H,11-14H2,1-3H3,(H,26,30)(H2,27,28,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of P38 mitogen activated protein kinase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
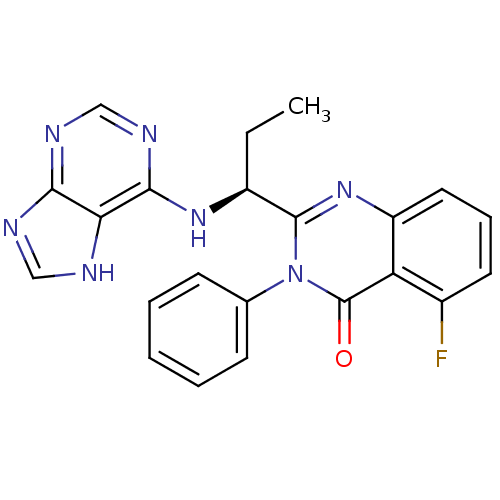
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of PIK3CD (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50268401
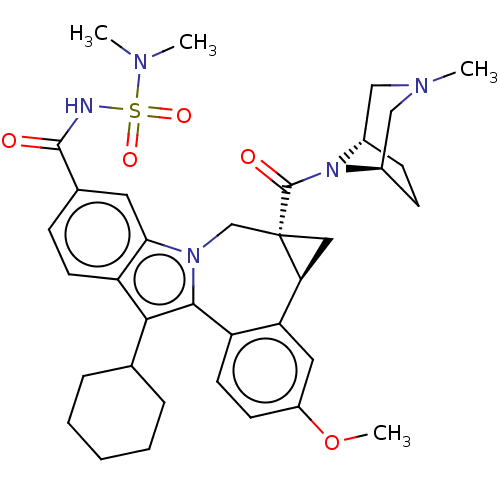
(CHEMBL4105584)Show SMILES [H][C@@]12C[C@@]1(Cn1c(c(C3CCCCC3)c3ccc(cc13)C(=O)NS(=O)(=O)N(C)C)-c1ccc(OC)cc21)C(=O)N1[C@@]2([H])CC[C@]1([H])CN(C)C2 |r,TLB:37:39:46.47.49:43.42,THB:48:47:39:43.42| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24-,25+,30-,36-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5b RNA polymerase |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50011036
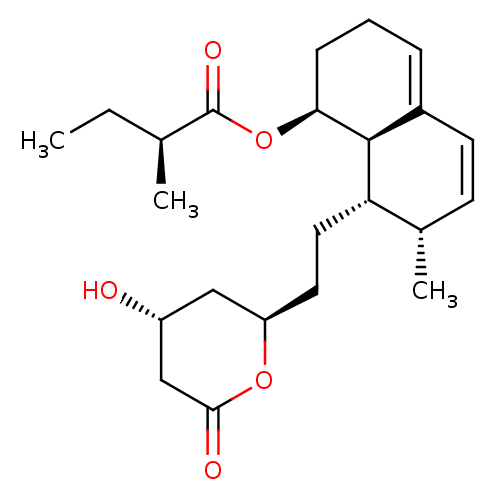
((S)-((1S,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxo-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1CCC=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:12,t:10| Show InChI InChI=1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA Reductase (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50505288
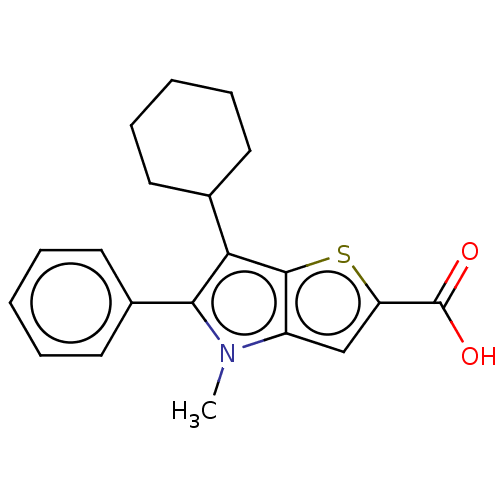
(CHEMBL1236660)Show InChI InChI=1S/C20H21NO2S/c1-21-15-12-16(20(22)23)24-19(15)17(13-8-4-2-5-9-13)18(21)14-10-6-3-7-11-14/h3,6-7,10-13H,2,4-5,8-9H2,1H3,(H,22,23) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5b RNA polymerase |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50439154
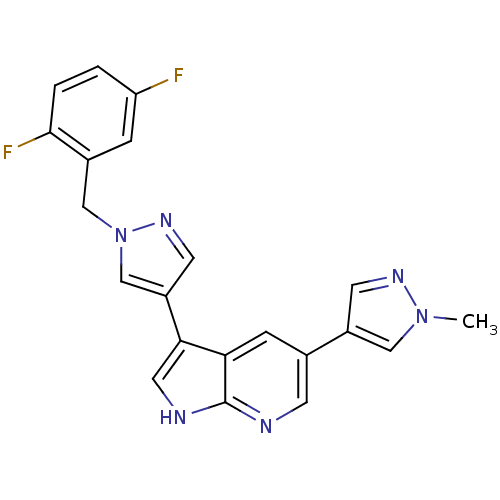
(CHEMBL2418761)Show SMILES Cn1cc(cn1)-c1cnc2[nH]cc(-c3cnn(Cc4cc(F)ccc4F)c3)c2c1 Show InChI InChI=1S/C21H16F2N6/c1-28-10-15(7-26-28)13-5-18-19(9-25-21(18)24-6-13)16-8-27-29(12-16)11-14-4-17(22)2-3-20(14)23/h2-10,12H,11H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50254585
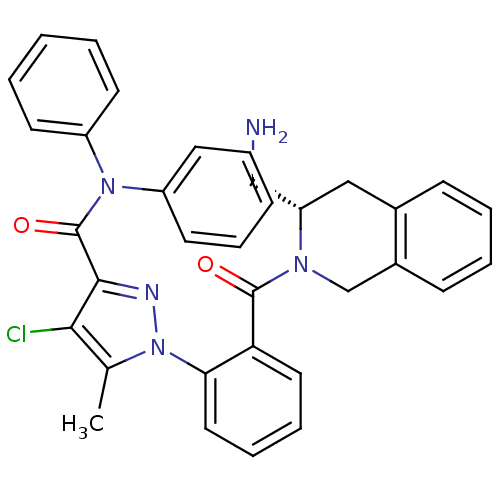
(1-(2-((S)-3-(aminomethyl)-1,2,3,4-tetrahydroisoqui...)Show SMILES Cc1c(Cl)c(nn1-c1ccccc1C(=O)N1Cc2ccccc2C[C@H]1CN)C(=O)N(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C34H30ClN5O2/c1-23-31(35)32(34(42)39(26-14-4-2-5-15-26)27-16-6-3-7-17-27)37-40(23)30-19-11-10-18-29(30)33(41)38-22-25-13-9-8-12-24(25)20-28(38)21-36/h2-19,28H,20-22,36H2,1H3/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50500146
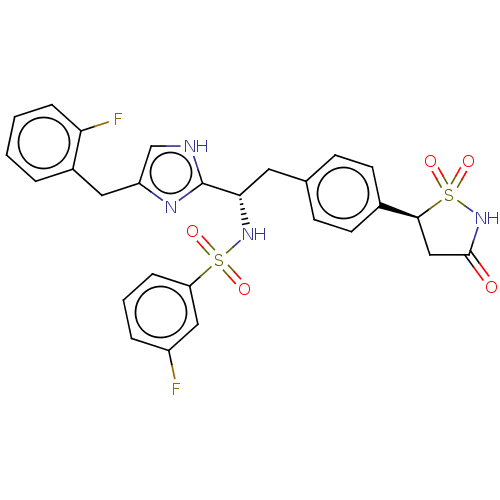
(CHEMBL3747361)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)c1nc(Cc2ccccc2F)c[nH]1 |r| Show InChI InChI=1S/C27H24F2N4O5S2/c28-20-5-3-6-22(14-20)39(35,36)32-24(27-30-16-21(31-27)13-19-4-1-2-7-23(19)29)12-17-8-10-18(11-9-17)25-15-26(34)33-40(25,37)38/h1-11,14,16,24-25,32H,12-13,15H2,(H,30,31)(H,33,34)/t24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50505287
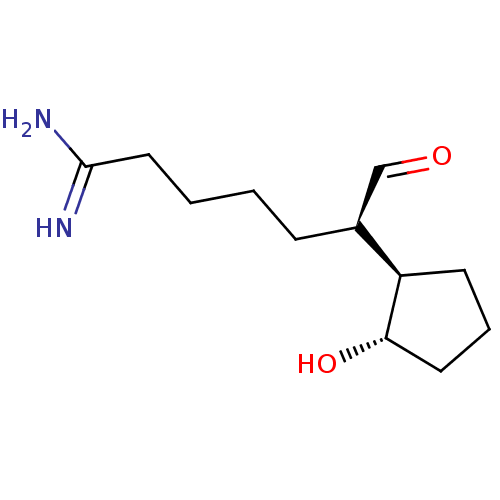
(CHEMBL1229657)Show InChI InChI=1S/C12H22N2O2/c13-12(14)7-2-1-4-9(8-15)10-5-3-6-11(10)16/h8-11,16H,1-7H2,(H3,13,14)/t9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50325999
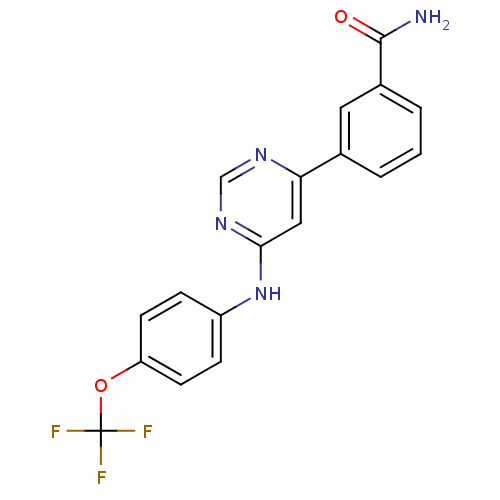
(3-(6-(4-(trifluoromethoxy)phenylamino)pyrimidin-4-...)Show SMILES NC(=O)c1cccc(c1)-c1cc(Nc2ccc(OC(F)(F)F)cc2)ncn1 Show InChI InChI=1S/C18H13F3N4O2/c19-18(20,21)27-14-6-4-13(5-7-14)25-16-9-15(23-10-24-16)11-2-1-3-12(8-11)17(22)26/h1-10H,(H2,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of wild-type BCR-ABL (unknown origin) expressed in mouse Ba/F3 cells as cell growth inhibition after 48 hrs by MTT assay |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50526695
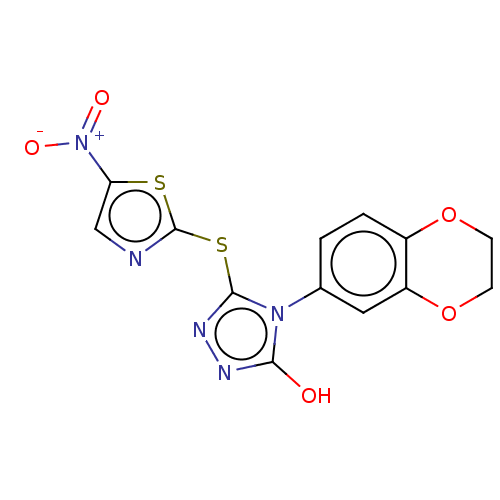
(BI-78D3 | CHEMBL508280)Show SMILES Oc1nnc(Sc2ncc(s2)[N+]([O-])=O)n1-c1ccc2OCCOc2c1 Show InChI InChI=1S/C13H9N5O5S2/c19-11-15-16-12(25-13-14-6-10(24-13)18(20)21)17(11)7-1-2-8-9(5-7)23-4-3-22-8/h1-2,5-6H,3-4H2,(H,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using ATF2 as substrate after 1 hr by Lanthascreen TR-FRET assay |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50173123
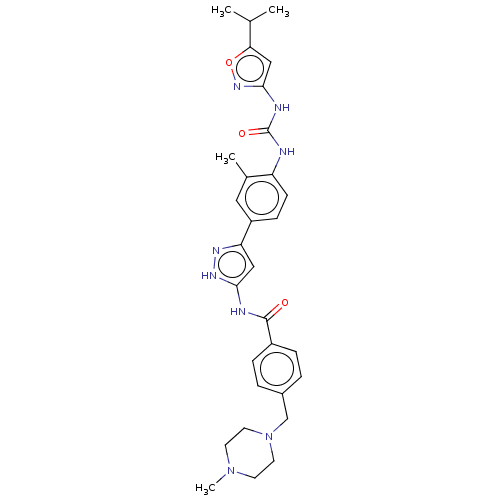
(CHEMBL3810133)Show SMILES CC(C)c1cc(NC(=O)Nc2ccc(cc2C)-c2cc(NC(=O)c3ccc(CN4CCN(C)CC4)cc3)[nH]n2)no1 Show InChI InChI=1S/C30H36N8O3/c1-19(2)26-17-28(36-41-26)33-30(40)31-24-10-9-23(15-20(24)3)25-16-27(35-34-25)32-29(39)22-7-5-21(6-8-22)18-38-13-11-37(4)12-14-38/h5-10,15-17,19H,11-14,18H2,1-4H3,(H2,31,33,36,40)(H2,32,34,35,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10005-10025 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01732
BindingDB Entry DOI: 10.7270/Q2ZG6WHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50526696

(CHEMBL4459106)Show SMILES CCCCCCCCCCCCCCCCCCOC[C@H](COP(O)(=O)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)C[C@H]1OCC1CCCCC1)OC |r| Show InChI InChI=1S/C35H69O10P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21-24-42-27-30(41-2)28-44-46(39,40)45-35-32(25-31(36)33(37)34(35)38)43-26-29-22-19-18-20-23-29/h29-38H,3-28H2,1-2H3,(H,39,40)/t30-,31-,32-,33+,34-,35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human P38alpha MAPK expressed in Escherichia coli Rosetta cells |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50526693
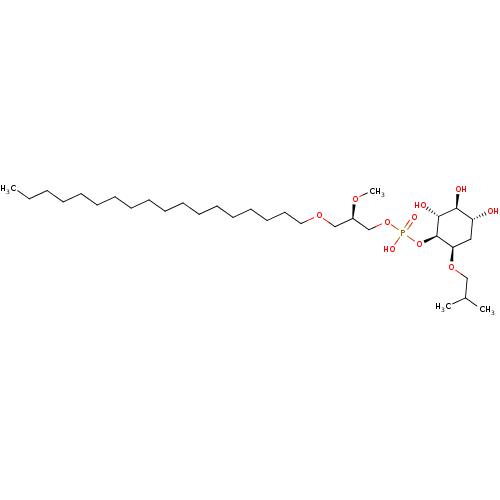
(CHEMBL4445946)Show SMILES CCCCCCCCCCCCCCCCCCOC[C@H](COP(O)(=O)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)C[C@H]1OCC(C)C)OC |r| Show InChI InChI=1S/C32H65O10P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-39-24-27(38-4)25-41-43(36,37)42-32-29(40-23-26(2)3)22-28(33)30(34)31(32)35/h26-35H,5-25H2,1-4H3,(H,36,37)/t27-,28-,29-,30+,31-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human P38alpha MAPK expressed in Escherichia coli Rosetta cells |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50431630
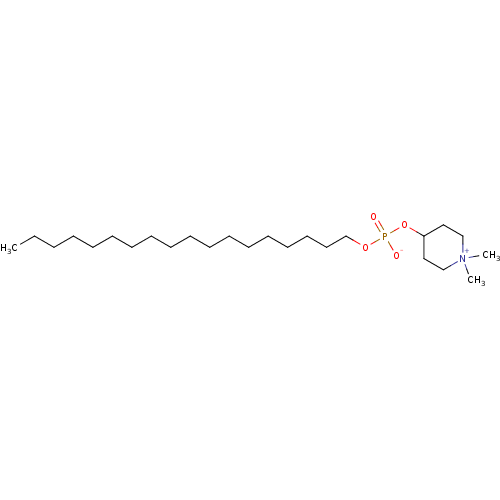
(KRX-0401 | PERIFOSINE)Show SMILES CCCCCCCCCCCCCCCCCCOP([O-])(=O)OC1CC[N+](C)(C)CC1 Show InChI InChI=1S/C25H52NO4P/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-24-29-31(27,28)30-25-20-22-26(2,3)23-21-25/h25H,4-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human P38alpha MAPK expressed in Escherichia coli Rosetta cells |
J Med Chem 62: 6512-6524 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00089
BindingDB Entry DOI: 10.7270/Q25X2DC4 |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50530042
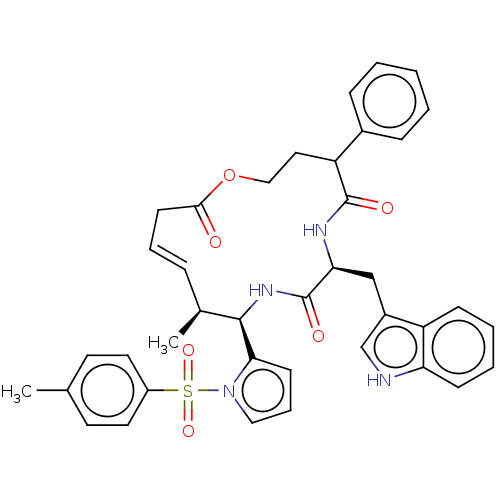
(CHEMBL4517868)Show SMILES C[C@H]1\C=C\CC(=O)OCCC(c2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H]1c1cccn1S(=O)(=O)c1ccc(C)cc1 |r,t:2| Show InChI InChI=1S/C39H40N4O6S/c1-26-17-19-30(20-18-26)50(47,48)43-22-9-15-35(43)37-27(2)10-8-16-36(44)49-23-21-32(28-11-4-3-5-12-28)38(45)41-34(39(46)42-37)24-29-25-40-33-14-7-6-13-31(29)33/h3-15,17-20,22,25,27,32,34,37,40H,16,21,23-24H2,1-2H3,(H,41,45)(H,42,46)/b10-8+/t27-,32?,34-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of full length human APE1 assessed as reduction in endonuclease activity by measuring DNA cleavage of abasic sites using 6-FAM labelled tw... |
J Med Chem 62: 1971-1988 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01529
BindingDB Entry DOI: 10.7270/Q2G73J6T |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50530042

(CHEMBL4517868)Show SMILES C[C@H]1\C=C\CC(=O)OCCC(c2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H]1c1cccn1S(=O)(=O)c1ccc(C)cc1 |r,t:2| Show InChI InChI=1S/C39H40N4O6S/c1-26-17-19-30(20-18-26)50(47,48)43-22-9-15-35(43)37-27(2)10-8-16-36(44)49-23-21-32(28-11-4-3-5-12-28)38(45)41-34(39(46)42-37)24-29-25-40-33-14-7-6-13-31(29)33/h3-15,17-20,22,25,27,32,34,37,40H,16,21,23-24H2,1-2H3,(H,41,45)(H,42,46)/b10-8+/t27-,32?,34-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of full length human APE1 assessed as reduction in endonuclease activity by measuring DNA cleavage of abasic sites using 6-FAM labelled tw... |
J Med Chem 62: 1971-1988 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01529
BindingDB Entry DOI: 10.7270/Q2G73J6T |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50530037
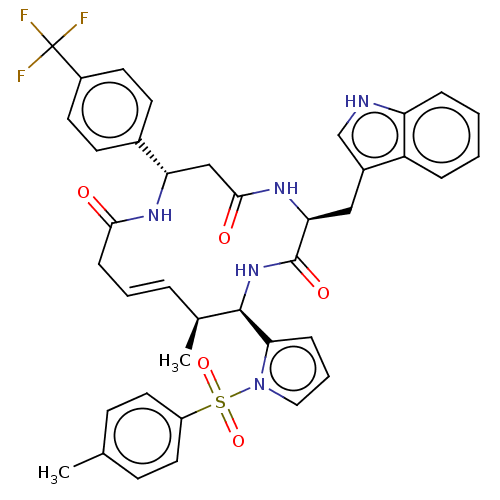
(CHEMBL4473257)Show SMILES C[C@H]1\C=C\CC(=O)N[C@@H](CC(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H]1c1cccn1S(=O)(=O)c1ccc(C)cc1)c1ccc(cc1)C(F)(F)F |r,t:2| Show InChI InChI=1S/C39H38F3N5O5S/c1-24-12-18-29(19-13-24)53(51,52)47-20-6-10-34(47)37-25(2)7-5-11-35(48)44-32(26-14-16-28(17-15-26)39(40,41)42)22-36(49)45-33(38(50)46-37)21-27-23-43-31-9-4-3-8-30(27)31/h3-10,12-20,23,25,32-33,37,43H,11,21-22H2,1-2H3,(H,44,48)(H,45,49)(H,46,50)/b7-5+/t25-,32-,33-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of full length human APE1 assessed as reduction in endonuclease activity by measuring DNA cleavage of abasic sites using 6-FAM labelled tw... |
J Med Chem 62: 1971-1988 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01529
BindingDB Entry DOI: 10.7270/Q2G73J6T |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50530037

(CHEMBL4473257)Show SMILES C[C@H]1\C=C\CC(=O)N[C@@H](CC(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H]1c1cccn1S(=O)(=O)c1ccc(C)cc1)c1ccc(cc1)C(F)(F)F |r,t:2| Show InChI InChI=1S/C39H38F3N5O5S/c1-24-12-18-29(19-13-24)53(51,52)47-20-6-10-34(47)37-25(2)7-5-11-35(48)44-32(26-14-16-28(17-15-26)39(40,41)42)22-36(49)45-33(38(50)46-37)21-27-23-43-31-9-4-3-8-30(27)31/h3-10,12-20,23,25,32-33,37,43H,11,21-22H2,1-2H3,(H,44,48)(H,45,49)(H,46,50)/b7-5+/t25-,32-,33-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of full length human APE1 assessed as reduction in endonuclease activity by measuring DNA cleavage of abasic sites using 6-FAM labelled tw... |
J Med Chem 62: 1971-1988 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01529
BindingDB Entry DOI: 10.7270/Q2G73J6T |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50530043
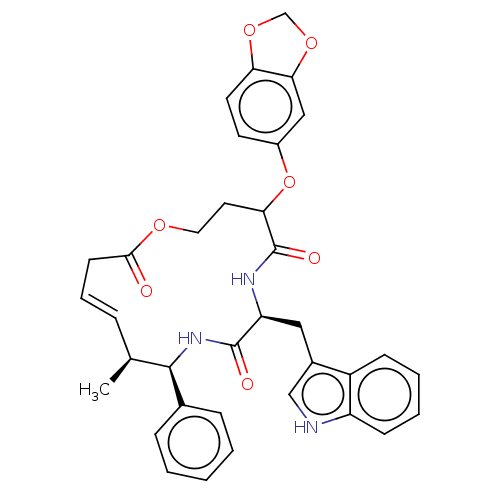
(CHEMBL4458126)Show SMILES C[C@H]1\C=C\CC(=O)OCCC(Oc2ccc3OCOc3c2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H]1c1ccccc1 |r,t:2| Show InChI InChI=1S/C35H35N3O7/c1-22-8-7-13-32(39)42-17-16-30(45-25-14-15-29-31(19-25)44-21-43-29)35(41)37-28(18-24-20-36-27-12-6-5-11-26(24)27)34(40)38-33(22)23-9-3-2-4-10-23/h2-12,14-15,19-20,22,28,30,33,36H,13,16-18,21H2,1H3,(H,37,41)(H,38,40)/b8-7+/t22-,28-,30?,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of full length human APE1 assessed as reduction in endonuclease activity by measuring DNA cleavage of abasic sites using 6-FAM labelled tw... |
J Med Chem 62: 1971-1988 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01529
BindingDB Entry DOI: 10.7270/Q2G73J6T |
More data for this
Ligand-Target Pair | |
DNA-(apurinic or apyrimidinic site) endonuclease
(Homo sapiens (Human)) | BDBM50530043

(CHEMBL4458126)Show SMILES C[C@H]1\C=C\CC(=O)OCCC(Oc2ccc3OCOc3c2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H]1c1ccccc1 |r,t:2| Show InChI InChI=1S/C35H35N3O7/c1-22-8-7-13-32(39)42-17-16-30(45-25-14-15-29-31(19-25)44-21-43-29)35(41)37-28(18-24-20-36-27-12-6-5-11-26(24)27)34(40)38-33(22)23-9-3-2-4-10-23/h2-12,14-15,19-20,22,28,30,33,36H,13,16-18,21H2,1H3,(H,37,41)(H,38,40)/b8-7+/t22-,28-,30?,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University
Curated by ChEMBL
| Assay Description
Inhibition of full length human APE1 assessed as reduction in endonuclease activity by measuring DNA cleavage of abasic sites using 6-FAM labelled tw... |
J Med Chem 62: 1971-1988 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01529
BindingDB Entry DOI: 10.7270/Q2G73J6T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data