

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50135997 (CHEMBL3754471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Competitive irreversible inhibition of human placental microsome aromatase using varying levels of [1beta3H]-androstenedione as substrate measured af... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135997 (CHEMBL3754471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Competitive reversible inhibition of human placental microsome aromatase using varying levels of [1beta3H]-androstenedione as substrate measured afte... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50398447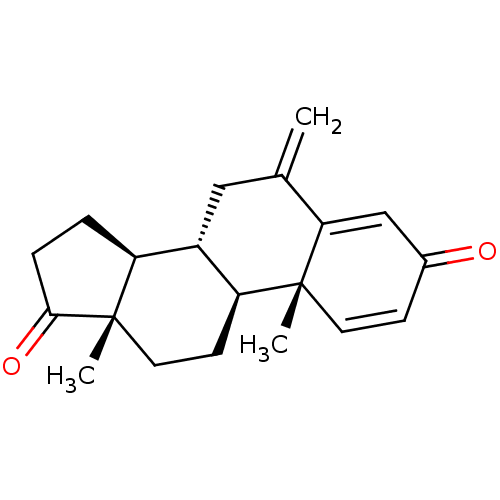 (Aromasin | EXEMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Competitive inhibition of human placental microsome aromatase using varying levels of [1beta2beta3H]-androstenedione as substrate | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50398447 (Aromasin | EXEMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523404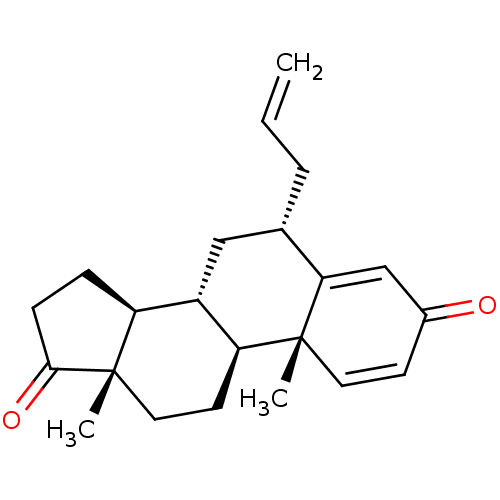 (CHEMBL4575994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135997 (CHEMBL3754471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069753 (CHEMBL3407538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523395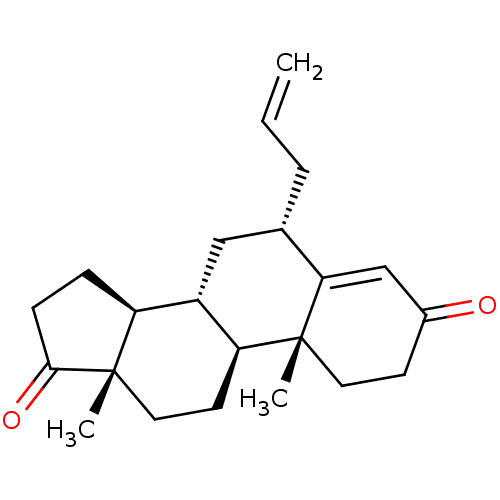 (CHEMBL4514728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523399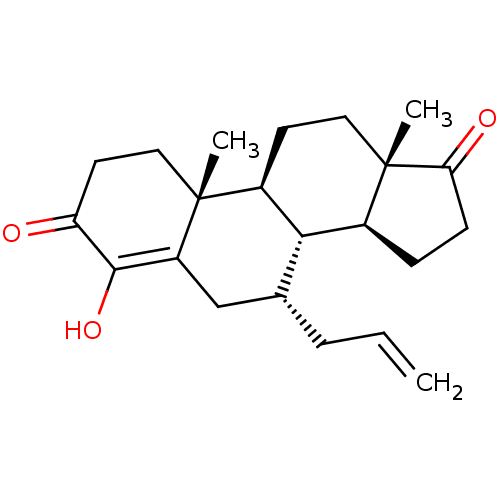 (CHEMBL4454575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50168058 (CHEMBL3798461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50135862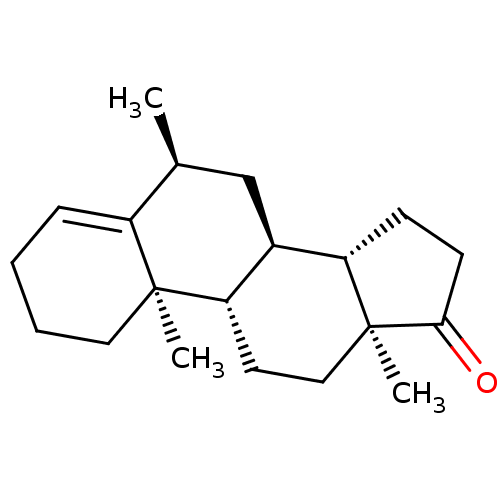 (CHEMBL3751881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523403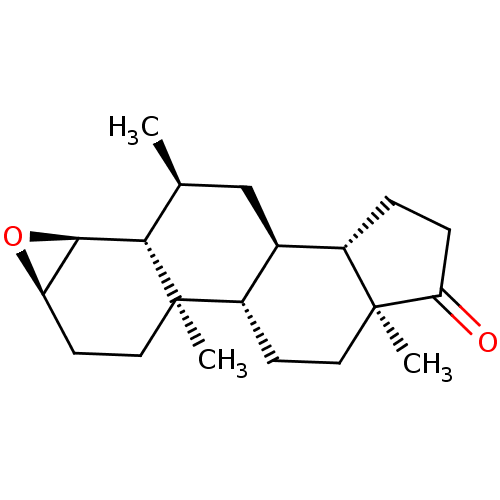 (CHEMBL4460869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523400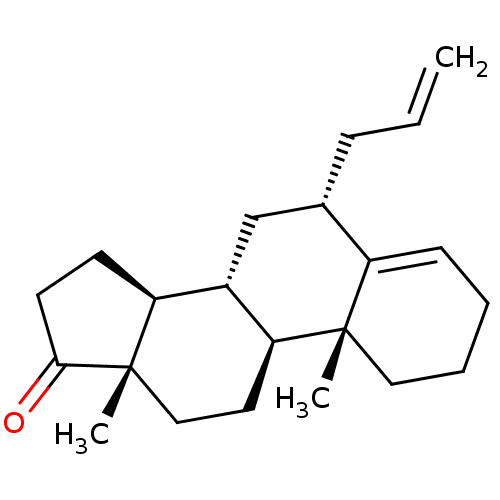 (CHEMBL4520056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069753 (CHEMBL3407538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50168056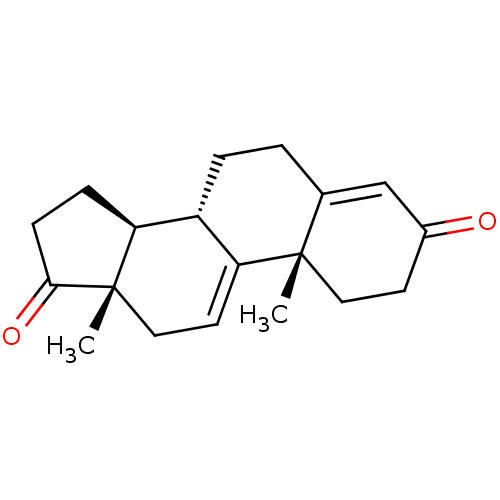 (CHEMBL3799363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523396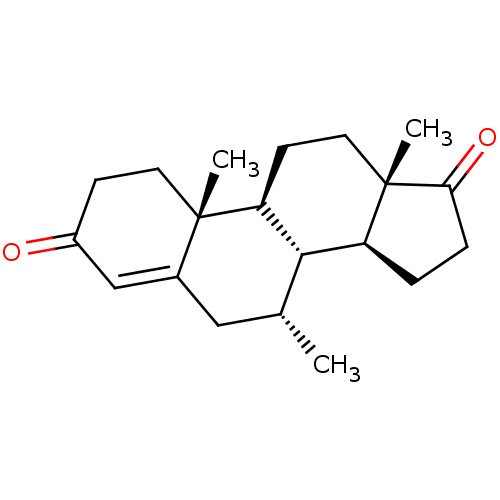 (CHEMBL487113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523397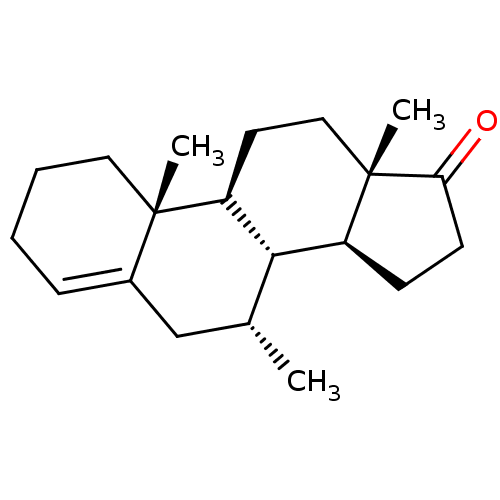 (CHEMBL4451962) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 405 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50123151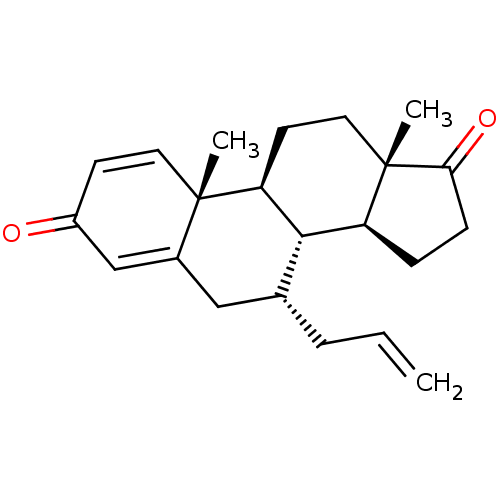 (CHEMBL3623223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523402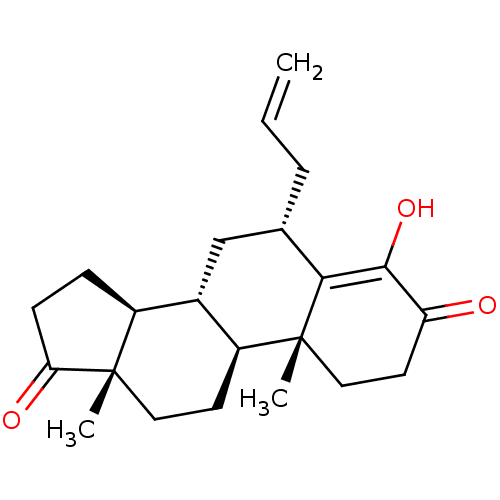 (CHEMBL4468497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50168057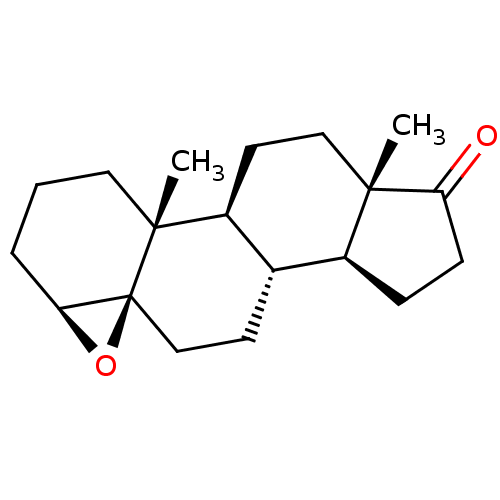 (CHEMBL3800417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of aromatase in human placental microsomes using [1beta-3H]-androstenedione as substrate assessed as tritiated H2O release after 15 mins b... | Bioorg Med Chem 24: 2823-31 (2016) Article DOI: 10.1016/j.bmc.2016.04.056 BindingDB Entry DOI: 10.7270/Q2H70HRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523398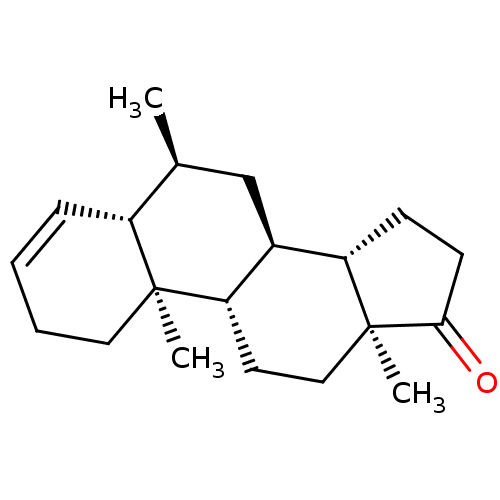 (CHEMBL4564333) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069755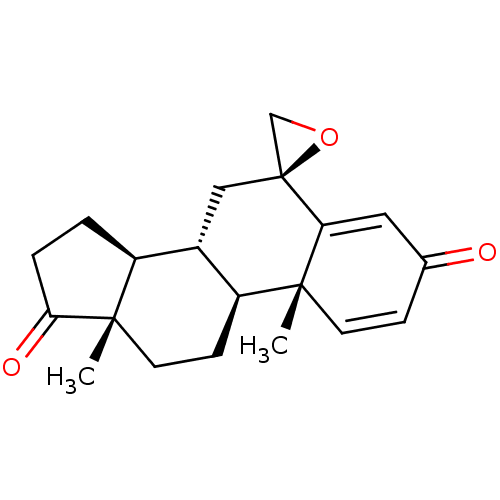 (CHEMBL3407536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069752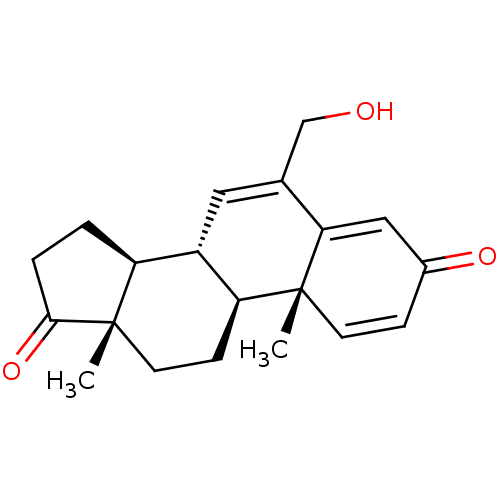 (CHEMBL3407539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069755 (CHEMBL3407536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069754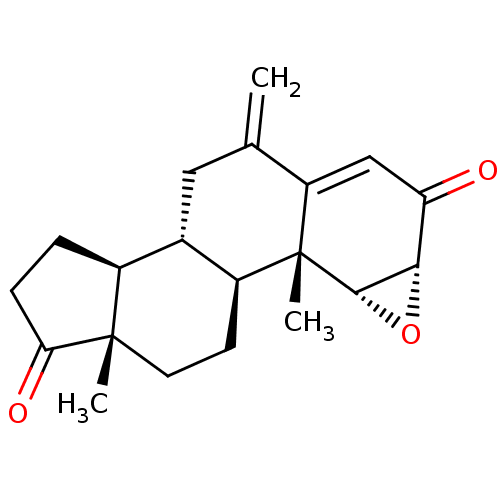 (CHEMBL3407537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta-3H] androstenedione as substrate after 15 mins by liquid scintillation counting | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523401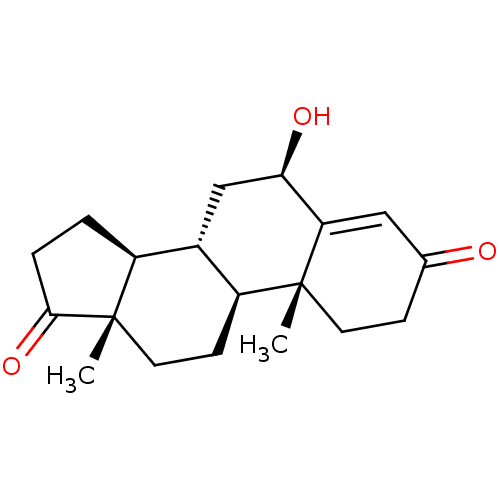 (CHEMBL4515880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50523405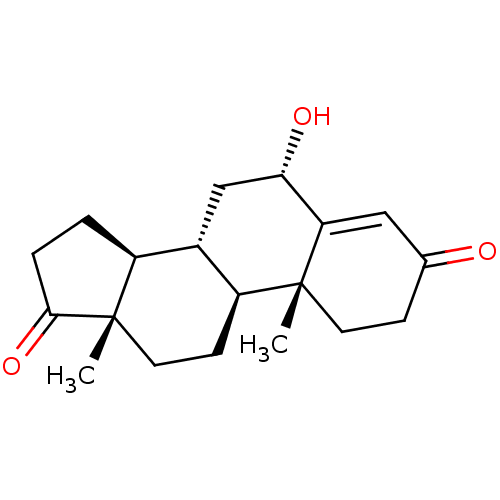 (CHEMBL4520027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta3H]-androstenedione as substrate measured after 15 mins in presence of NADPH by liquid ... | J Med Chem 62: 3636-3657 (2019) Article DOI: 10.1021/acs.jmedchem.9b00157 BindingDB Entry DOI: 10.7270/Q2N301CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50398447 (Aromasin | EXEMESTANE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069752 (CHEMBL3407539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50069754 (CHEMBL3407537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase expressed in human MCF7 cells using [1beta-3H] androstenedione as substrate after 1 hr by liquid sc... | Eur J Med Chem 87: 336-45 (2014) Article DOI: 10.1016/j.ejmech.2014.09.074 BindingDB Entry DOI: 10.7270/Q2JH3NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||