 Found 260 hits with Last Name = 'hayashi' and Initial = 't'
Found 260 hits with Last Name = 'hayashi' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50444438
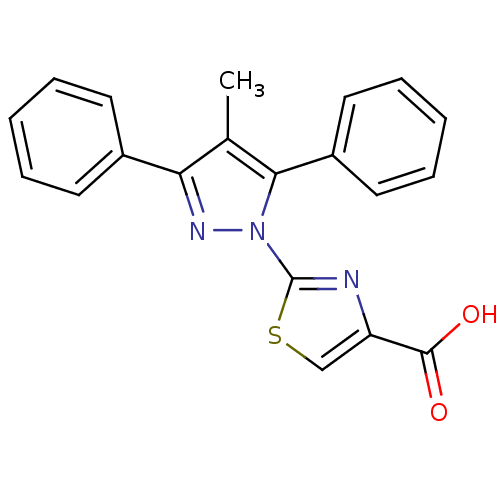
(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493969
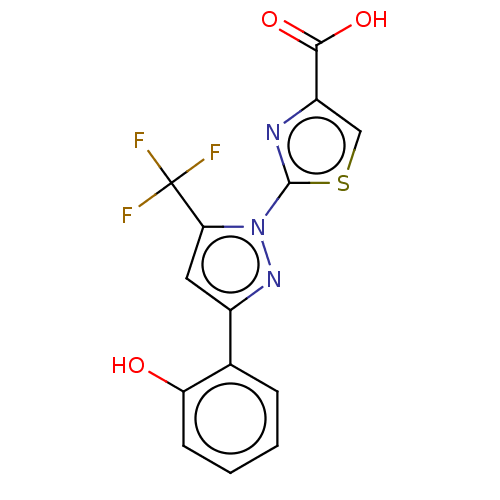
(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50449137
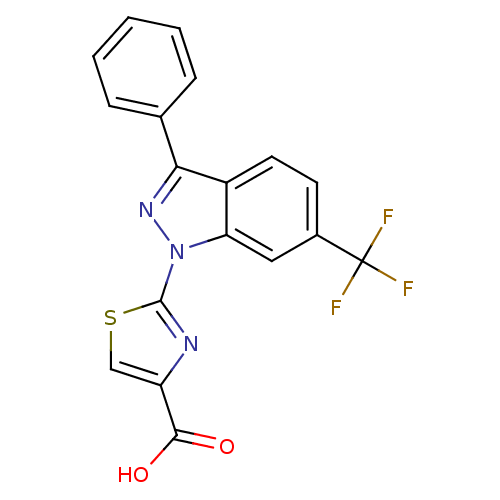
(CHEMBL3127163)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(cc12)C(F)(F)F Show InChI InChI=1S/C18H10F3N3O2S/c19-18(20,21)11-6-7-12-14(8-11)24(17-22-13(9-27-17)16(25)26)23-15(12)10-4-2-1-3-5-10/h1-9H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276250
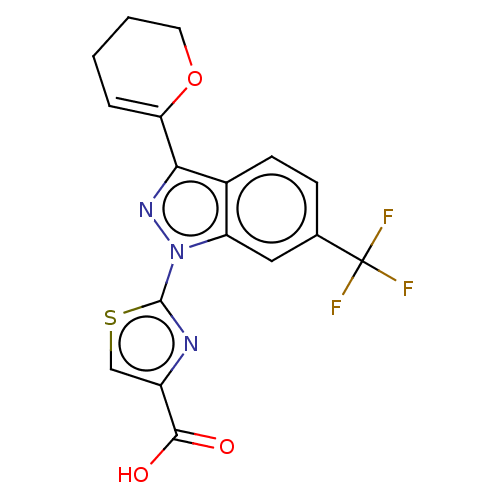
(CHEMBL4126319)Show SMILES OC(=O)c1csc(n1)-n1nc(C2=CCCCO2)c2ccc(cc12)C(F)(F)F |t:12| Show InChI InChI=1S/C17H12F3N3O3S/c18-17(19,20)9-4-5-10-12(7-9)23(16-21-11(8-27-16)15(24)25)22-14(10)13-3-1-2-6-26-13/h3-5,7-8H,1-2,6H2,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ACE by fluorometric assay |
J Nat Prod 51: 357-359 (1988)
Article DOI: 10.1021/np50056a033
BindingDB Entry DOI: 10.7270/Q2XW4M1C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276264
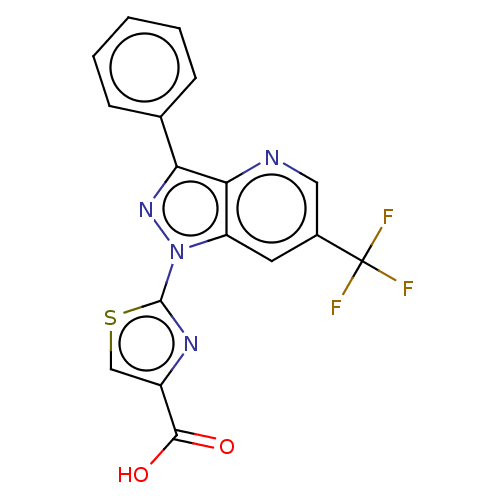
(CHEMBL4128163)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ncc(cc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)10-6-12-14(21-7-10)13(9-4-2-1-3-5-9)23-24(12)16-22-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276265
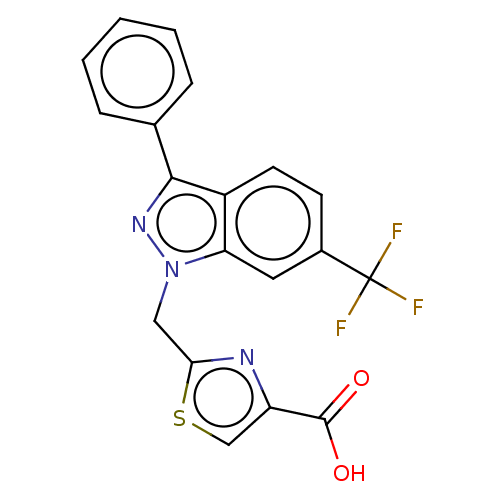
(CHEMBL4129545)Show SMILES OC(=O)c1csc(Cn2nc(-c3ccccc3)c3ccc(cc23)C(F)(F)F)n1 Show InChI InChI=1S/C19H12F3N3O2S/c20-19(21,22)12-6-7-13-15(8-12)25(9-16-23-14(10-28-16)18(26)27)24-17(13)11-4-2-1-3-5-11/h1-8,10H,9H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276263
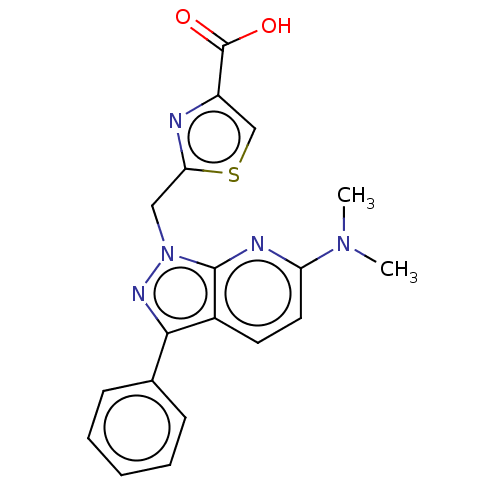
(CHEMBL4129401)Show SMILES CN(C)c1ccc2c(nn(Cc3nc(cs3)C(O)=O)c2n1)-c1ccccc1 Show InChI InChI=1S/C19H17N5O2S/c1-23(2)15-9-8-13-17(12-6-4-3-5-7-12)22-24(18(13)21-15)10-16-20-14(11-27-16)19(25)26/h3-9,11H,10H2,1-2H3,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276249
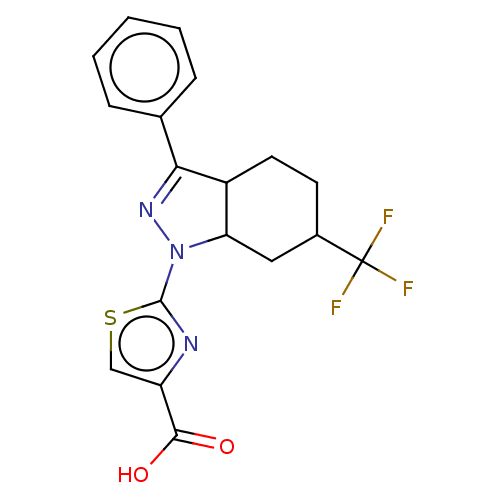
(CHEMBL4126167)Show SMILES OC(=O)c1csc(n1)N1N=C(C2CCC(CC12)C(F)(F)F)c1ccccc1 |c:10| Show InChI InChI=1S/C18H16F3N3O2S/c19-18(20,21)11-6-7-12-14(8-11)24(17-22-13(9-27-17)16(25)26)23-15(12)10-4-2-1-3-5-10/h1-5,9,11-12,14H,6-8H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50292535
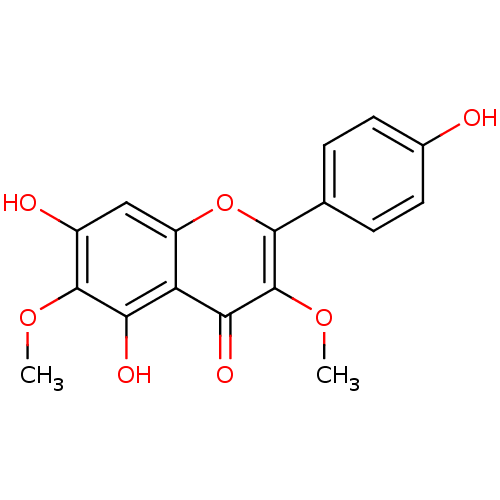
(5,7-Dihydroxy-2-(4-hydroxy-phenyl)-3,6-dimethoxy-c...)Show InChI InChI=1S/C17H14O7/c1-22-16-10(19)7-11-12(13(16)20)14(21)17(23-2)15(24-11)8-3-5-9(18)6-4-8/h3-7,18-20H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cow milk xanthine oxidase |
J Nat Prod 51: 345-348 (1988)
Article DOI: 10.1021/np50056a030
BindingDB Entry DOI: 10.7270/Q2DJ5FNN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276266
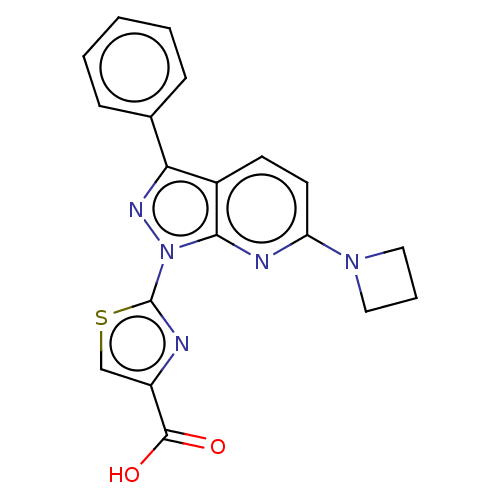
(CHEMBL4126096)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)N1CCC1 Show InChI InChI=1S/C19H15N5O2S/c25-18(26)14-11-27-19(20-14)24-17-13(7-8-15(21-17)23-9-4-10-23)16(22-24)12-5-2-1-3-6-12/h1-3,5-8,11H,4,9-10H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493974

(CHEMBL2442494)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1C(F)(F)F)-c1ccccc1 Show InChI InChI=1S/C15H10F3N3O2S/c1-8-11(9-5-3-2-4-6-9)20-21(12(8)15(16,17)18)14-19-10(7-24-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50444437
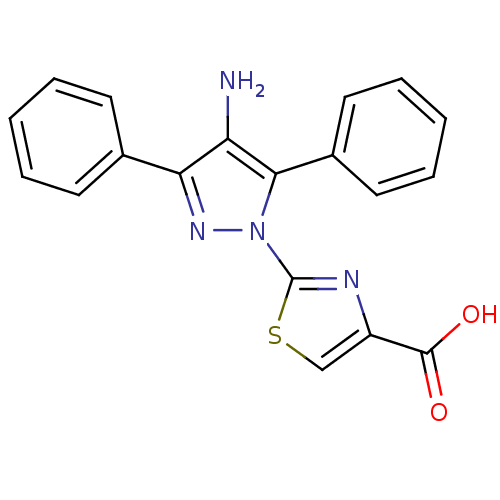
(CHEMBL3092130)Show SMILES Nc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C19H14N4O2S/c20-15-16(12-7-3-1-4-8-12)22-23(17(15)13-9-5-2-6-10-13)19-21-14(11-26-19)18(24)25/h1-11H,20H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of TP receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6569-76 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.065
BindingDB Entry DOI: 10.7270/Q2BR8TMB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50250685
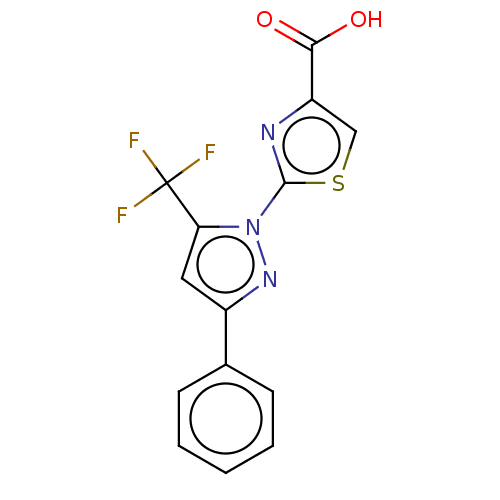
(CHEMBL1479261 | US10961200, Compound 62 | US112479...)Show InChI InChI=1S/C14H8F3N3O2S/c15-14(16,17)11-6-9(8-4-2-1-3-5-8)19-20(11)13-18-10(7-23-13)12(21)22/h1-7H,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493970
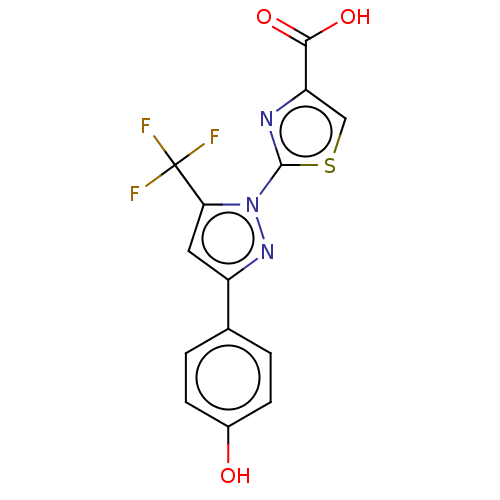
(CHEMBL2442488)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccc(O)cc1 Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-9(7-1-3-8(21)4-2-7)19-20(11)13-18-10(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493971
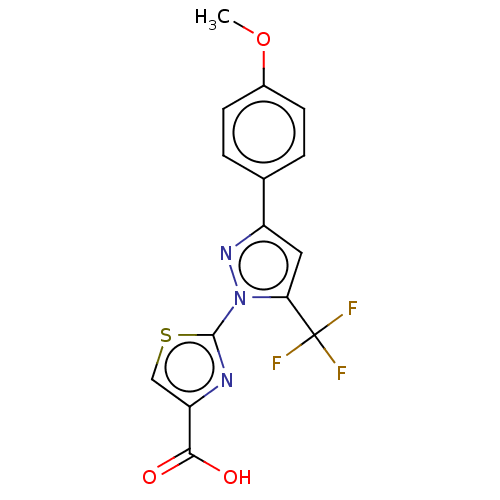
(CHEMBL2442486)Show SMILES COc1ccc(cc1)-c1cc(n(n1)-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)10-6-12(15(16,17)18)21(20-10)14-19-11(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by intracellular Ca2+ release assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493972
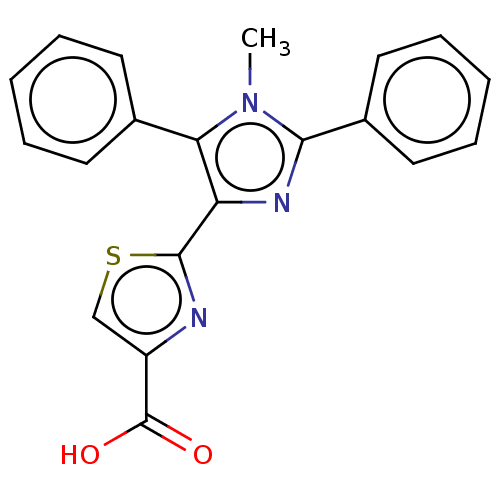
(CHEMBL2442497)Show SMILES Cn1c(nc(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-23-17(13-8-4-2-5-9-13)16(19-21-15(12-26-19)20(24)25)22-18(23)14-10-6-3-7-11-14/h2-12H,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493973
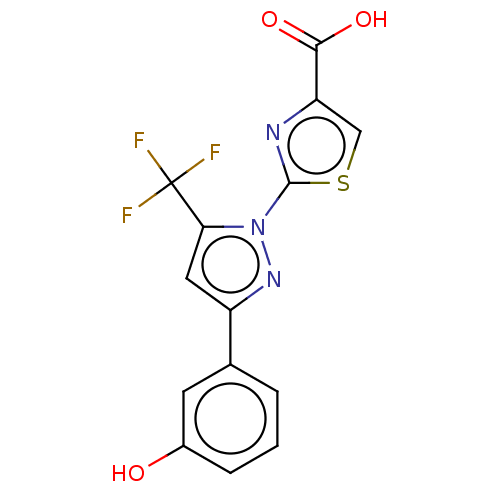
(CHEMBL2442489)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1cccc(O)c1 Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-9(7-2-1-3-8(21)4-7)19-20(11)13-18-10(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50041529
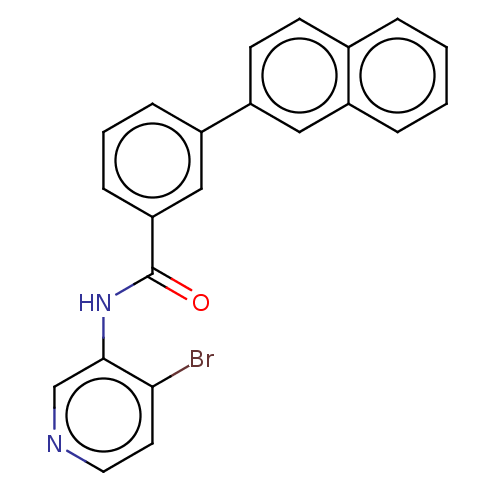
(CHEMBL3358391)Show InChI InChI=1S/C22H15BrN2O/c23-20-10-11-24-14-21(20)25-22(26)19-7-3-6-17(13-19)18-9-8-15-4-1-2-5-16(15)12-18/h1-14H,(H,25,26) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant SIRT2 (34 to 356 residues) expressed in Escherichia coli BL21 using Fluor de Lys-SIRT as substrate incubat... |
Bioorg Med Chem 23: 328-39 (2015)
Article DOI: 10.1016/j.bmc.2014.11.027
BindingDB Entry DOI: 10.7270/Q2736SJS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by intracellular Ca2+ release assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50041522
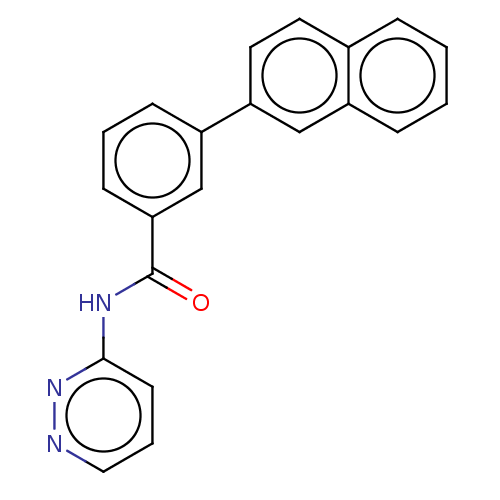
(CHEMBL3358386)Show InChI InChI=1S/C21H15N3O/c25-21(23-20-9-4-12-22-24-20)19-8-3-7-17(14-19)18-11-10-15-5-1-2-6-16(15)13-18/h1-14H,(H,23,24,25) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant SIRT2 (34 to 356 residues) expressed in Escherichia coli BL21 using Fluor de Lys-SIRT as substrate incubat... |
Bioorg Med Chem 23: 328-39 (2015)
Article DOI: 10.1016/j.bmc.2014.11.027
BindingDB Entry DOI: 10.7270/Q2736SJS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50000029
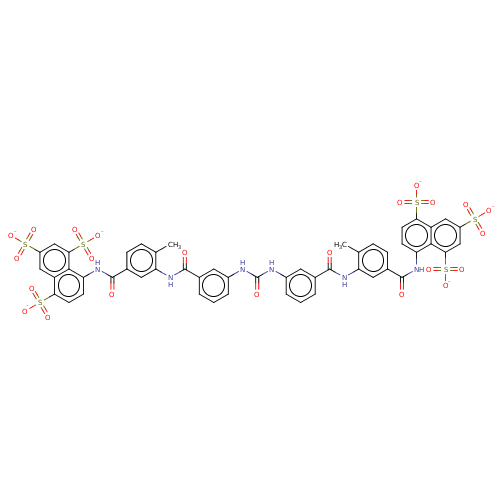
(4-Methyl-8-{4-methyl-3-[3-(3-{3-[2-methyl-5-(4,6,8...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/p-6 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SIRT2 |
Bioorg Med Chem 23: 328-39 (2015)
Article DOI: 10.1016/j.bmc.2014.11.027
BindingDB Entry DOI: 10.7270/Q2736SJS |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM35440
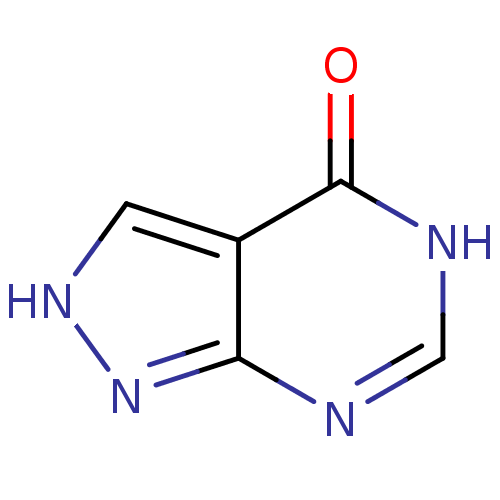
(ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...)Show InChI InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cow milk xanthine oxidase |
J Nat Prod 52: 210-211 (1989)
Article DOI: 10.1021/np50061a035
BindingDB Entry DOI: 10.7270/Q24749W5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP4 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50216689
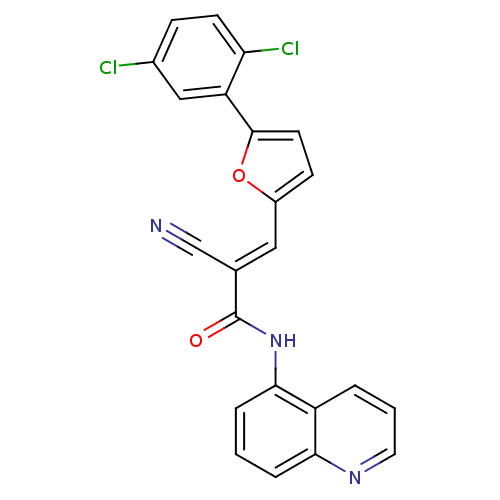
(2-cyano-3-(5-(2,5-dichlorophenyl)furan-2-yl)-N-(qu...)Show SMILES Clc1ccc(Cl)c(c1)-c1ccc(\C=C(/C#N)C(=O)Nc2cccc3ncccc23)o1 Show InChI InChI=1S/C23H13Cl2N3O2/c24-15-6-8-19(25)18(12-15)22-9-7-16(30-22)11-14(13-26)23(29)28-21-5-1-4-20-17(21)3-2-10-27-20/h1-12H,(H,28,29)/b14-11+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant SIRT2 (34 to 356 residues) expressed in Escherichia coli BL21 using Fluor de Lys-SIRT as substrate incubat... |
Bioorg Med Chem 23: 328-39 (2015)
Article DOI: 10.1016/j.bmc.2014.11.027
BindingDB Entry DOI: 10.7270/Q2736SJS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493975
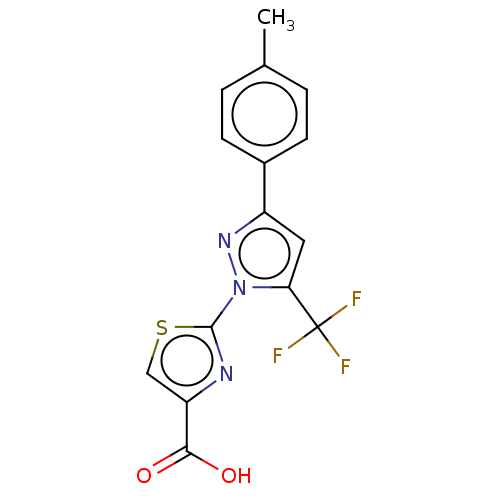
(CHEMBL2442487)Show SMILES Cc1ccc(cc1)-c1cc(n(n1)-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O2S/c1-8-2-4-9(5-3-8)10-6-12(15(16,17)18)21(20-10)14-19-11(7-24-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor by reporter gene assay |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50241052

(1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33-,34+,35-,41+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cow milk xanthine oxidase |
J Nat Prod 52: 210-211 (1989)
Article DOI: 10.1021/np50061a035
BindingDB Entry DOI: 10.7270/Q24749W5 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cow milk xanthine oxidase |
J Nat Prod 51: 345-348 (1988)
Article DOI: 10.1021/np50056a030
BindingDB Entry DOI: 10.7270/Q2DJ5FNN |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50041514
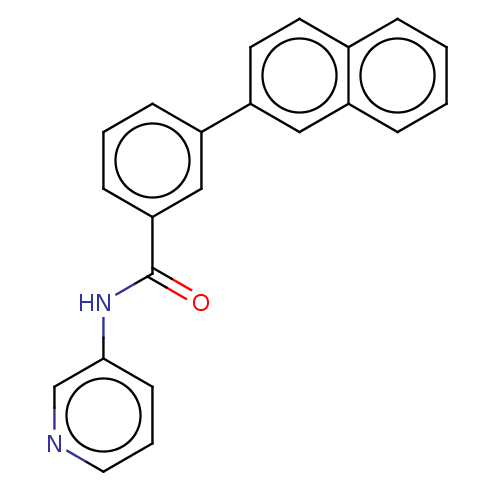
(CHEMBL3358382)Show InChI InChI=1S/C22H16N2O/c25-22(24-21-9-4-12-23-15-21)20-8-3-7-18(14-20)19-11-10-16-5-1-2-6-17(16)13-19/h1-15H,(H,24,25) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human recombinant SIRT2 (34 to 356 residues) expressed in Escherichia coli BL21 using Fluor de Lys-SIRT as substrate incubat... |
Bioorg Med Chem 23: 328-39 (2015)
Article DOI: 10.1016/j.bmc.2014.11.027
BindingDB Entry DOI: 10.7270/Q2736SJS |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50216689

(2-cyano-3-(5-(2,5-dichlorophenyl)furan-2-yl)-N-(qu...)Show SMILES Clc1ccc(Cl)c(c1)-c1ccc(\C=C(/C#N)C(=O)Nc2cccc3ncccc23)o1 Show InChI InChI=1S/C23H13Cl2N3O2/c24-15-6-8-19(25)18(12-15)22-9-7-16(30-22)11-14(13-26)23(29)28-21-5-1-4-20-17(21)3-2-10-27-20/h1-12H,(H,28,29)/b14-11+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SIRT2 |
Bioorg Med Chem 23: 328-39 (2015)
Article DOI: 10.1016/j.bmc.2014.11.027
BindingDB Entry DOI: 10.7270/Q2736SJS |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50033767
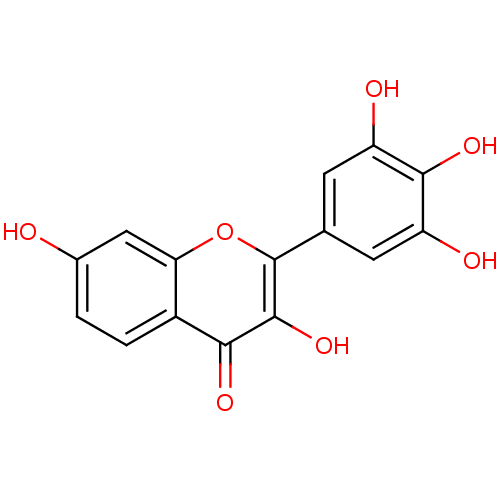
(3,3',4',5',7-pentahydroxy flavone | 3,7,3',4',5'-P...)Show InChI InChI=1S/C15H10O7/c16-7-1-2-8-11(5-7)22-15(14(21)12(8)19)6-3-9(17)13(20)10(18)4-6/h1-5,16-18,20-21H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cow milk xanthine oxidase |
J Nat Prod 51: 345-348 (1988)
Article DOI: 10.1021/np50056a030
BindingDB Entry DOI: 10.7270/Q2DJ5FNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data