

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171495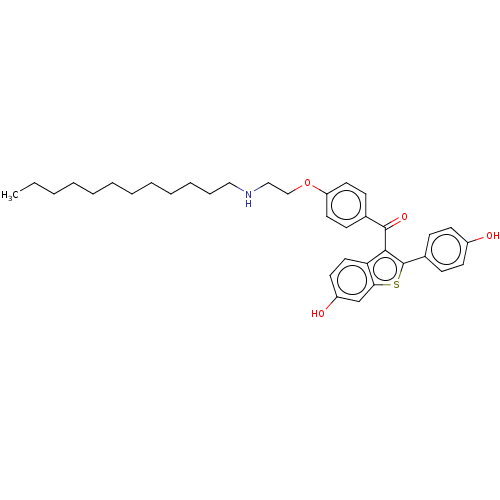 (CHEMBL3805318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at ERalpha (unknown origin) transfected in 17beta-estradiol induced-HEK293 cells assessed as inhibition of estradiol-mediated pro... | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171494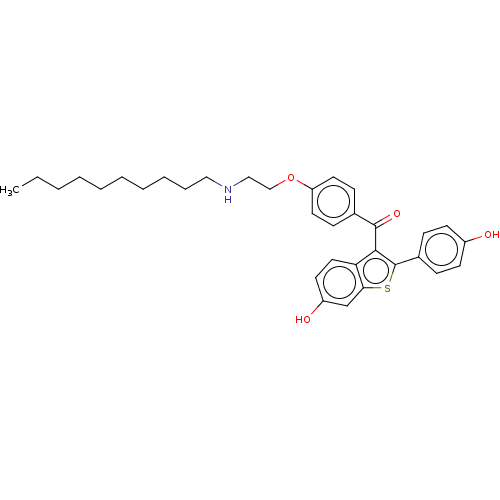 (CHEMBL3806167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at ERalpha (unknown origin) transfected in 17beta-estradiol induced-HEK293 cells assessed as inhibition of estradiol-mediated pro... | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171499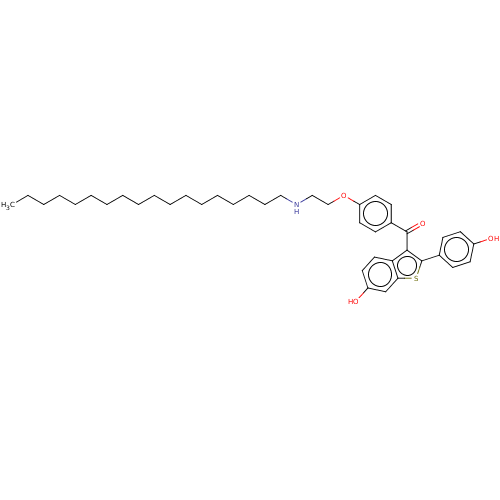 (CHEMBL3805385) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50437046 (CHEMBL2403356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in Hog plasma | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50437046 (CHEMBL2403356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha (unknown origin) expressed in human MCF7 cells assessed as inhibition of E2-induced response by dual luciferase report... | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171495 (CHEMBL3805318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50437046 (CHEMBL2403356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from full length recombinant human untagged ERalpha expressed in Sf insect cells by fluorescence polarization assay | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171498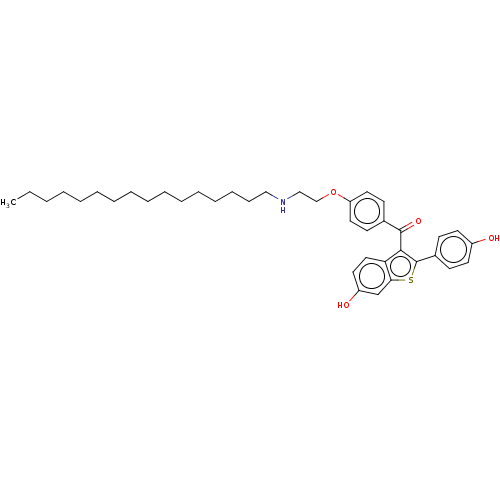 (CHEMBL3805025) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104834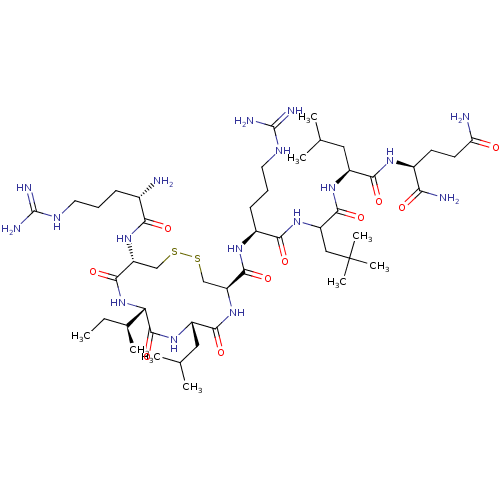 (CHEMBL3597499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171497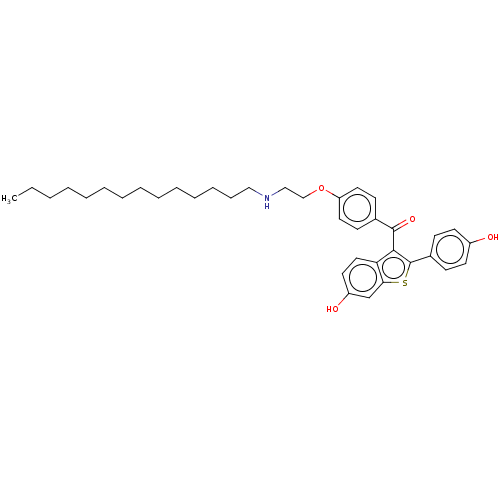 (CHEMBL3804907) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Mus musculus) | BDBM20608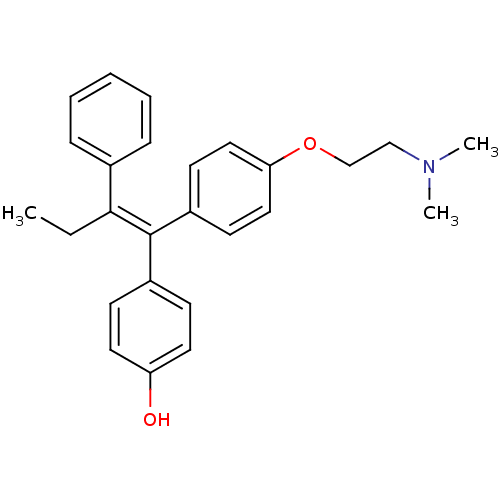 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in mouse GT1-7 cells harboring beta-galactosidase reporter gene assessed as reduction in 17beta-estradiol in... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104835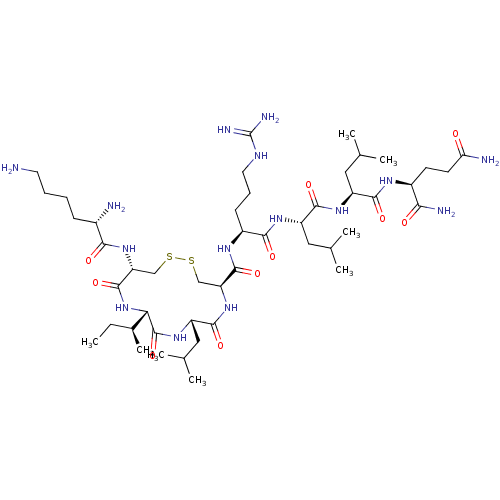 (CHEMBL3597498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171494 (CHEMBL3806167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171493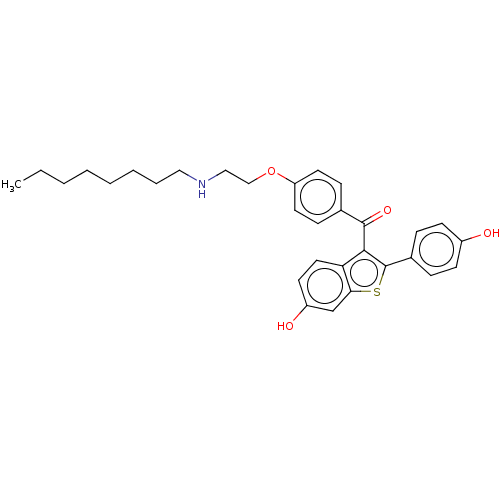 (CHEMBL3805170) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50171492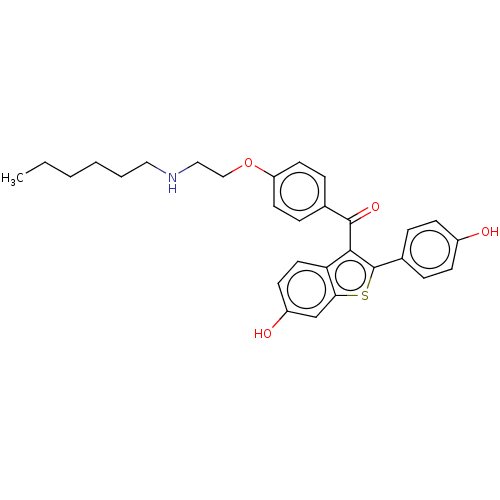 (CHEMBL3805921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay | Bioorg Med Chem 24: 2914-2919 (2016) Article DOI: 10.1016/j.bmc.2016.04.068 BindingDB Entry DOI: 10.7270/Q21V5GWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240314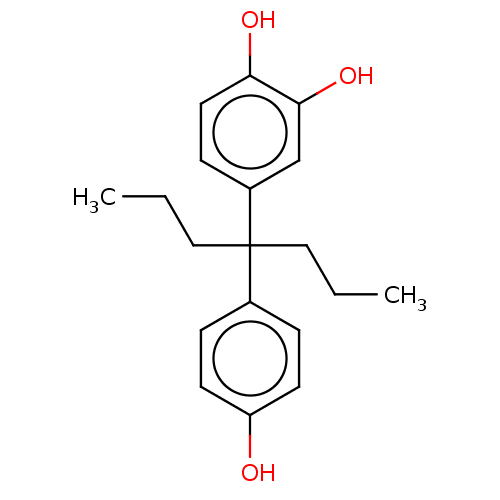 (CHEMBL4059760) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at CMX-GAL4N fused human ERalpha expressed in HEK293 cells assessed as reduction in E2 induced response by luciferase reporter ge... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104800 (CHEMBL3597509) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104804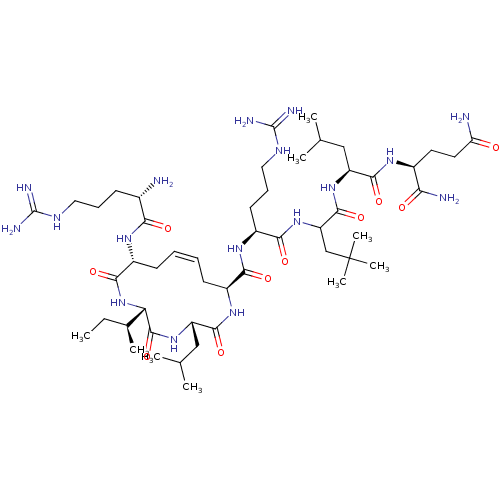 (CHEMBL3597505) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240313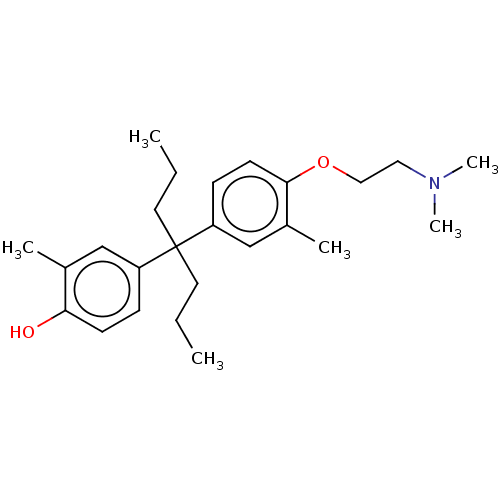 (CHEMBL4103516) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at CMX-GAL4N fused human ERalpha expressed in HEK293 cells assessed as reduction in E2 induced response by luciferase reporter ge... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240312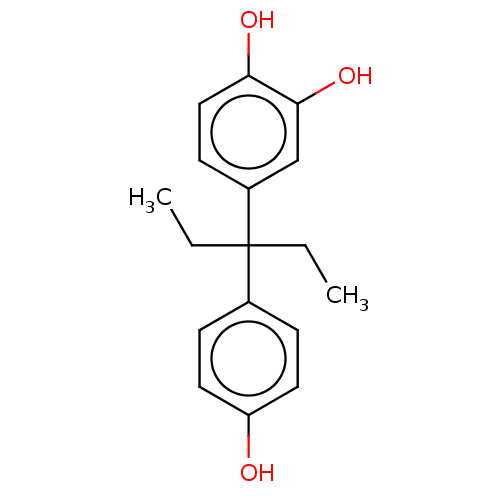 (CHEMBL4075785) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at CMX-GAL4N fused human ERalpha expressed in HEK293 cells assessed as reduction in E2 induced response by luciferase reporter ge... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240310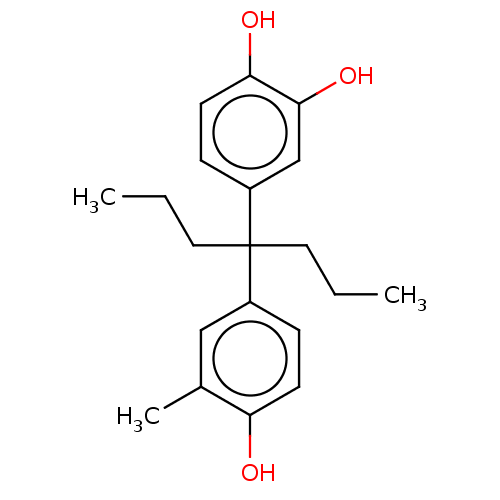 (CHEMBL4080172) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at CMX-GAL4N fused human ERalpha expressed in HEK293 cells assessed as reduction in E2 induced response by luciferase reporter ge... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50460592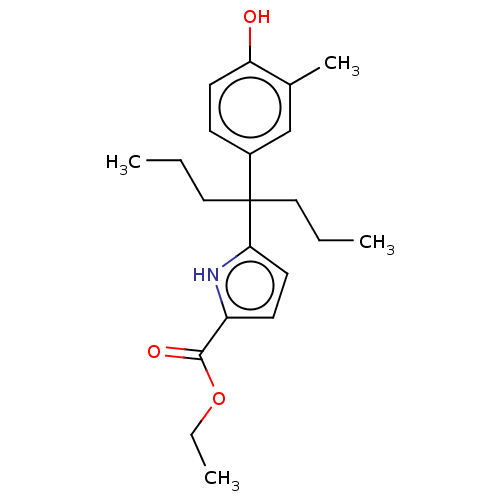 (CHEMBL4225034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from full length recombinant human untagged ERalpha expressed in Sf insect cells by fluorescence polarization assay | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240309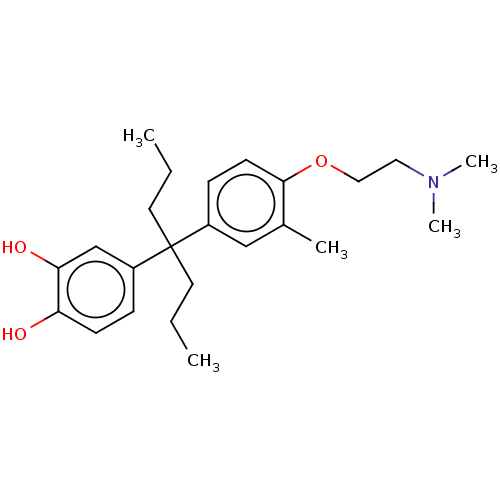 (CHEMBL4098201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at CMX-GAL4N fused human ERalpha expressed in HEK293 cells assessed as reduction in E2 induced response by luciferase reporter ge... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240308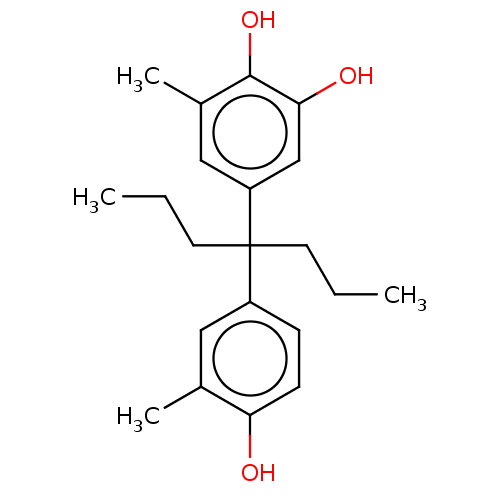 (CHEMBL4079256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Antagonist activity at CMX-GAL4N fused human ERalpha expressed in HEK293 cells assessed as reduction in E2 induced response by luciferase reporter ge... | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104805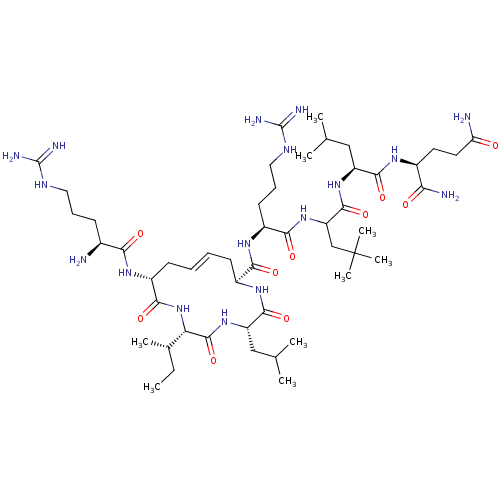 (CHEMBL3597504) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50460592 (CHEMBL4225034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Antagonist activity at ERalpha (unknown origin) expressed in human MCF7 cells assessed as inhibition of E2-induced response by dual luciferase report... | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50460598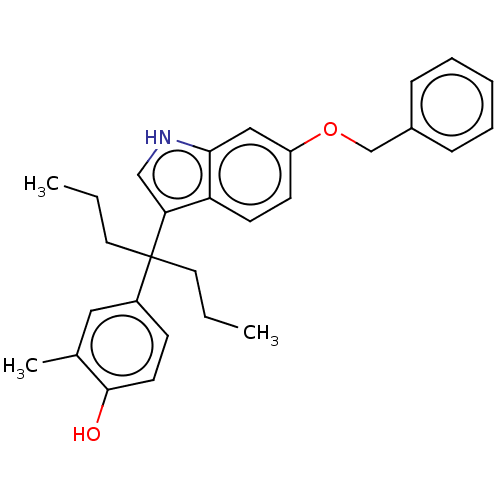 (CHEMBL4226152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from full length recombinant human untagged ERalpha expressed in Sf insect cells by fluorescence polarization assay | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104808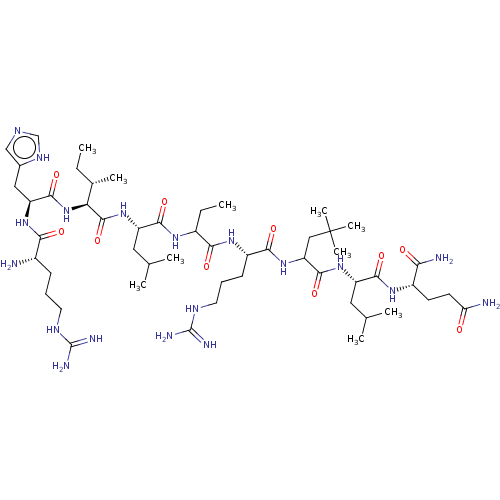 (CHEMBL3597501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50240311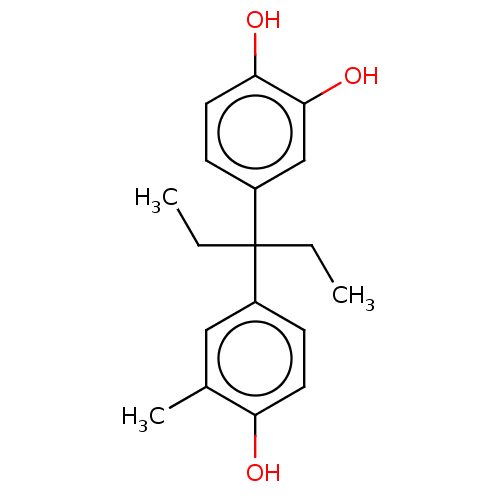 (CHEMBL4088044) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 664 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in Hog plasma | Bioorg Med Chem Lett 27: 2590-2593 (2017) Article DOI: 10.1016/j.bmcl.2017.03.066 BindingDB Entry DOI: 10.7270/Q2S184N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104807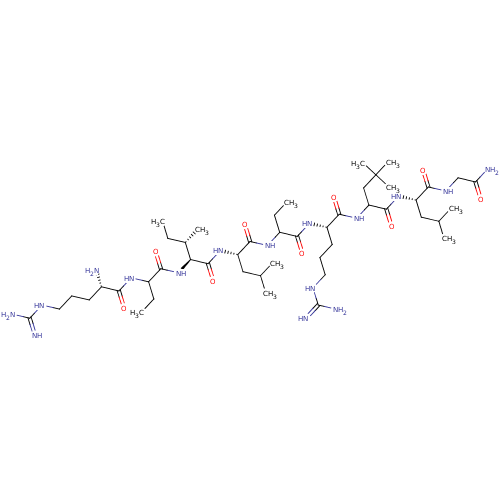 (CHEMBL3597502) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50012275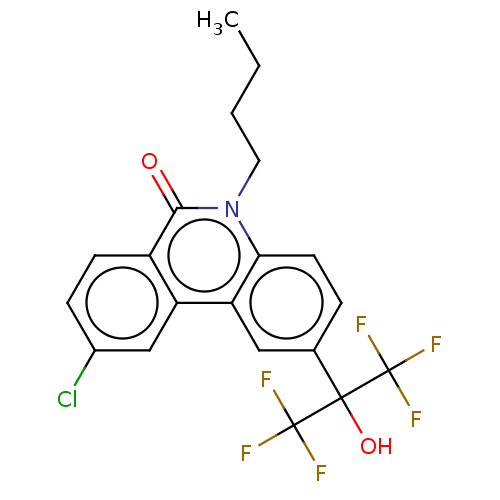 (CHEMBL3260000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human LXR-beta expressed in HEK293 cells assessed as inhibition of T0901317-induced effect after 16 hrs by luciferase reporter... | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50012275 (CHEMBL3260000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inverse agonist activity at human ROR-gamma1 expressed in HEK293 cells after 16 hrs by luciferase reporter gene assay | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104801 (CHEMBL3597508) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 749 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50012274 (CHEMBL3259999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inverse agonist activity at human ROR-gamma1 expressed in HEK293 cells after 16 hrs by luciferase reporter gene assay | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104797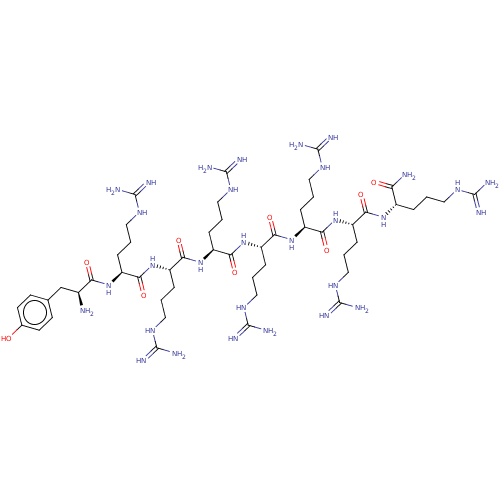 (CHEMBL3597514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104809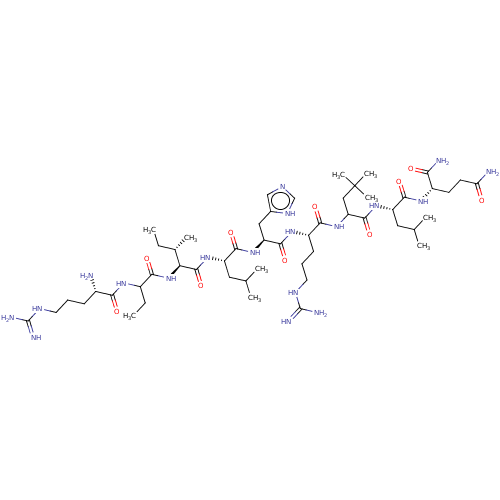 (CHEMBL3597500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104806 (CHEMBL3597503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104798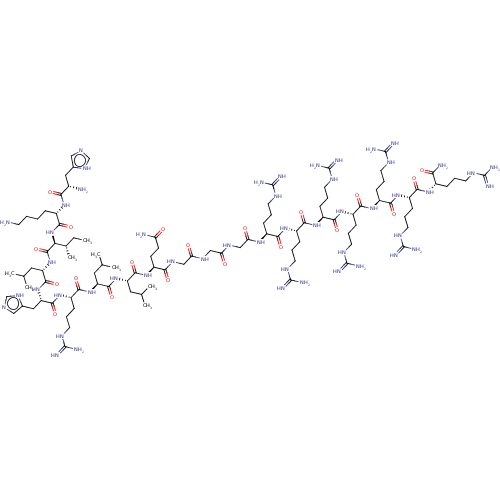 (CHEMBL3597513) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104803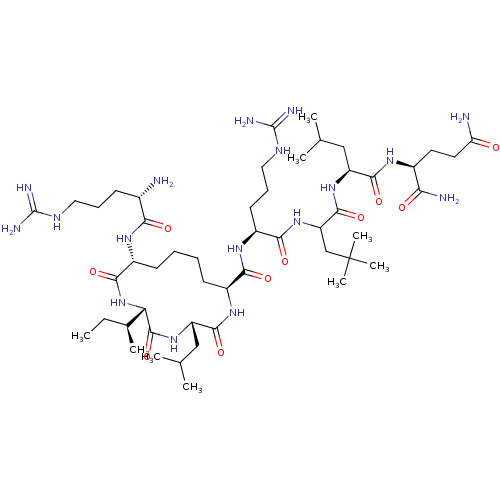 (CHEMBL3597506) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104799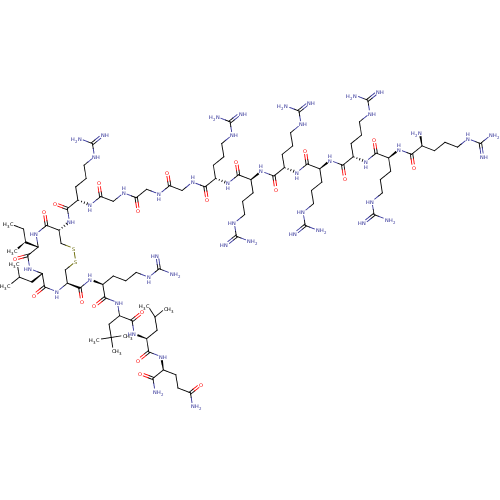 (CHEMBL3597510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50104802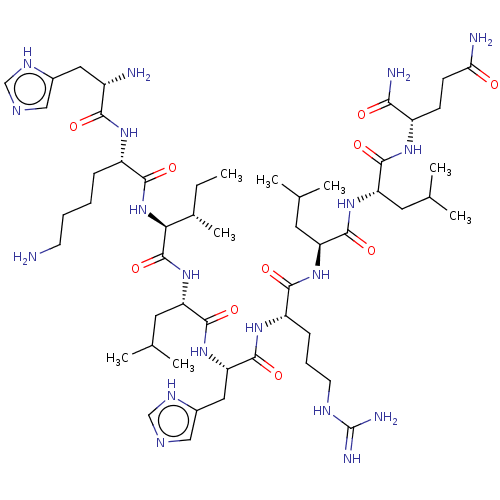 (CHEMBL3597507) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method | Bioorg Med Chem 23: 4132-8 (2015) Article DOI: 10.1016/j.bmc.2015.06.067 BindingDB Entry DOI: 10.7270/Q2V989T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50460594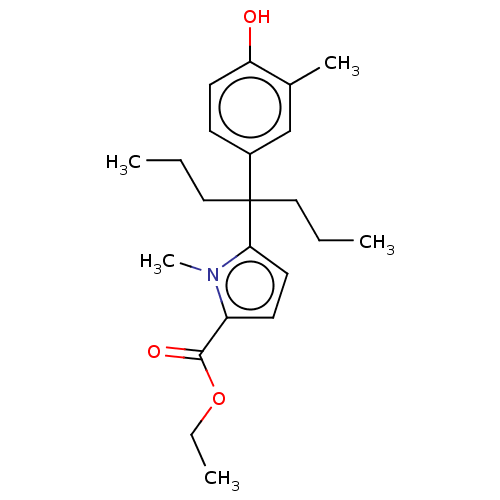 (CHEMBL4225619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from full length recombinant human untagged ERalpha expressed in Sf insect cells by fluorescence polarization assay | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50012273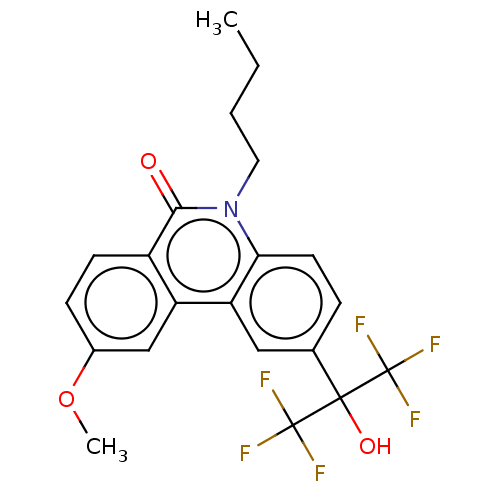 (CHEMBL1765174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inverse agonist activity at human ROR-gamma1 expressed in HEK293 cells after 16 hrs by luciferase reporter gene assay | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50460597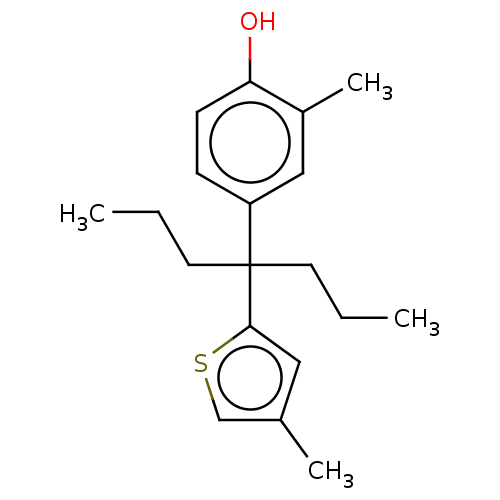 (CHEMBL4225888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Displacement of Fluormone ES2 from full length recombinant human untagged ERalpha expressed in Sf insect cells by fluorescence polarization assay | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50012276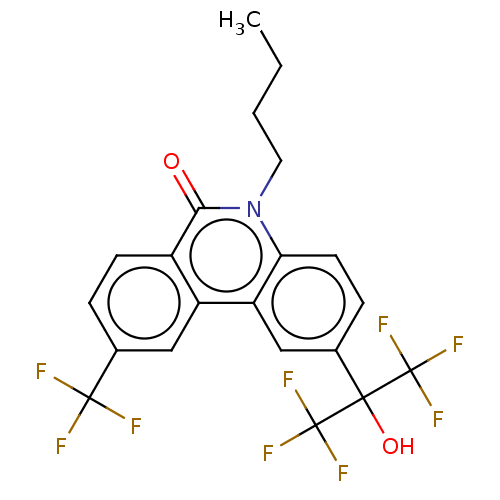 (CHEMBL3260001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inverse agonist activity at human ROR-gamma1 expressed in HEK293 cells after 16 hrs by luciferase reporter gene assay | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50012275 (CHEMBL3260000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human LXR-alpha expressed in HEK293 cells assessed as inhibition of T0901317-induced effect after 16 hrs by luciferase reporte... | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50012260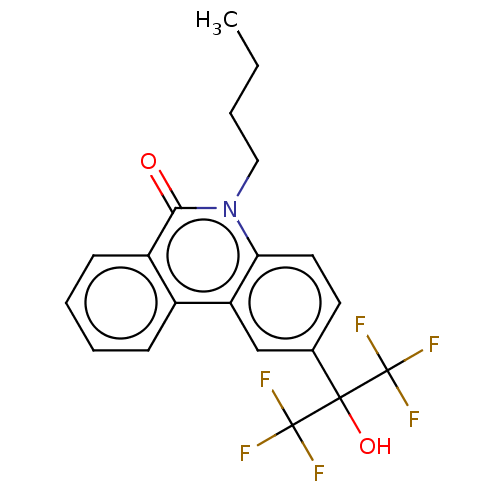 (CHEMBL1091939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inverse agonist activity at human ROR-gamma1 expressed in HEK293 cells after 16 hrs by luciferase reporter gene assay | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50012277 (CHEMBL3260002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inverse agonist activity at human ROR-gamma1 expressed in HEK293 cells after 16 hrs by luciferase reporter gene assay | Bioorg Med Chem 22: 2799-808 (2014) Article DOI: 10.1016/j.bmc.2014.03.007 BindingDB Entry DOI: 10.7270/Q28K7BN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 166 total ) | Next | Last >> |