

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257123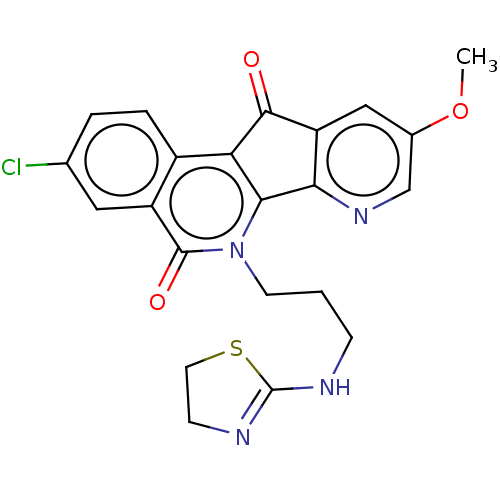 (CHEMBL4072279) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257089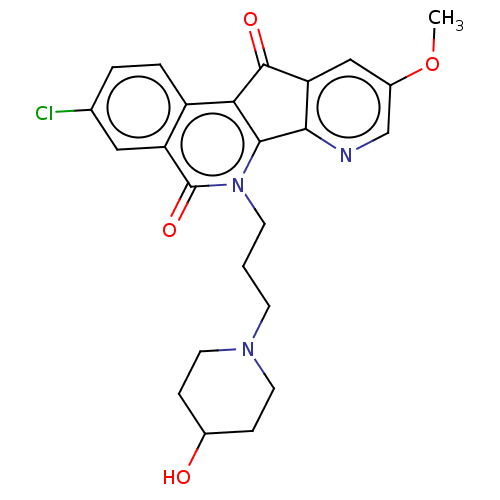 (CHEMBL4083140) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257106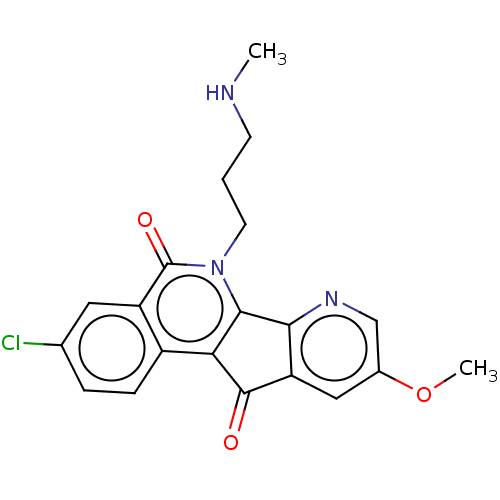 (CHEMBL4077196) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257102 (CHEMBL4090922) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257107 (CHEMBL4079315) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257120 (CHEMBL4072940) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257123 (CHEMBL4072279) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257105 (CHEMBL4064072) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257101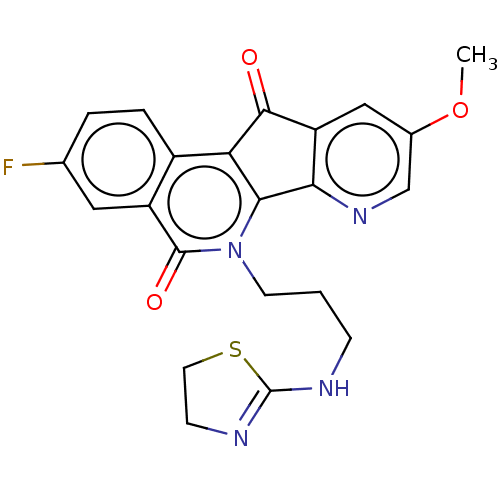 (CHEMBL4063586) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257119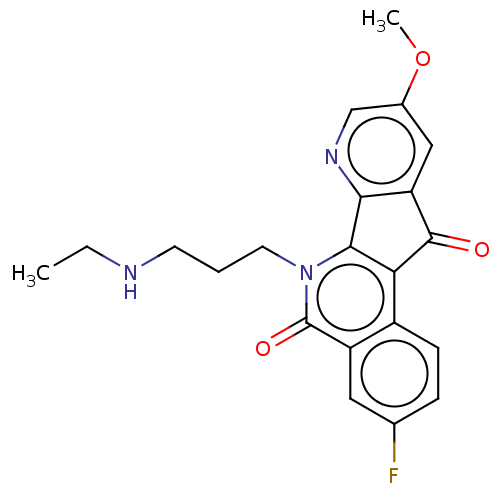 (CHEMBL4076362) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257104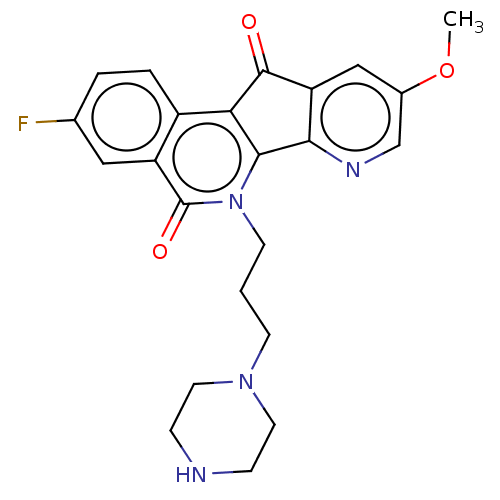 (CHEMBL4063011) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257124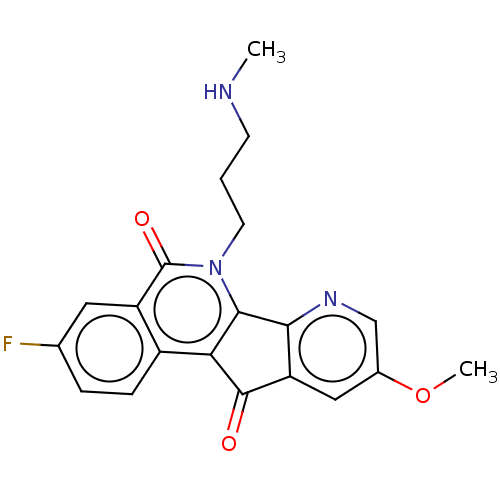 (CHEMBL4070873) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257126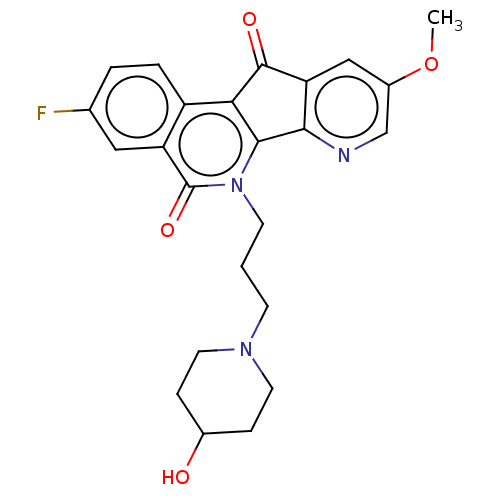 (CHEMBL4101133) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257127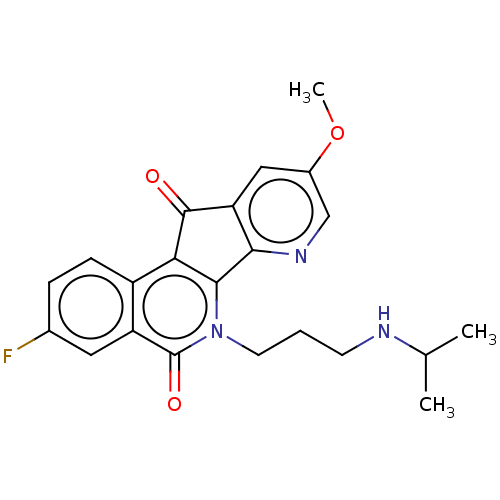 (CHEMBL4085641) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257124 (CHEMBL4070873) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257125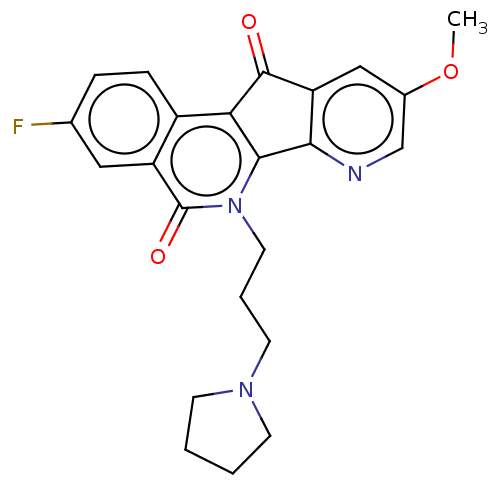 (CHEMBL4094373) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257099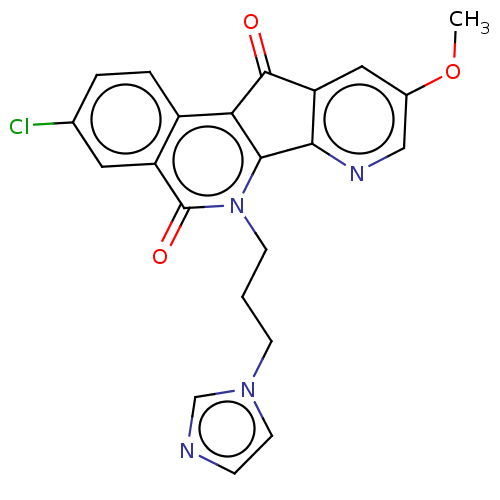 (CHEMBL4103520) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257126 (CHEMBL4101133) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257127 (CHEMBL4085641) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50008923 ((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257089 (CHEMBL4083140) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257090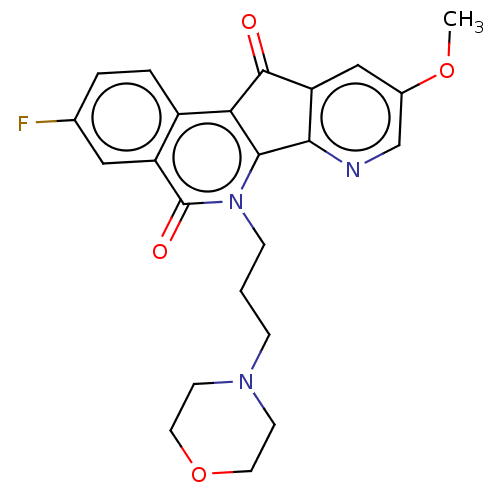 (CHEMBL4072994) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257099 (CHEMBL4103520) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257100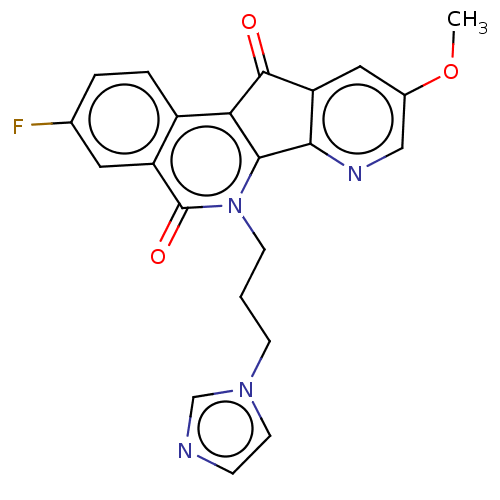 (CHEMBL4079995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257101 (CHEMBL4063586) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257104 (CHEMBL4063011) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257119 (CHEMBL4076362) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257122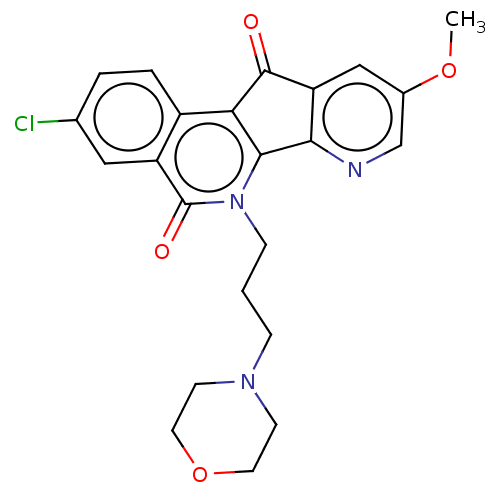 (CHEMBL4099136) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50008923 ((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257121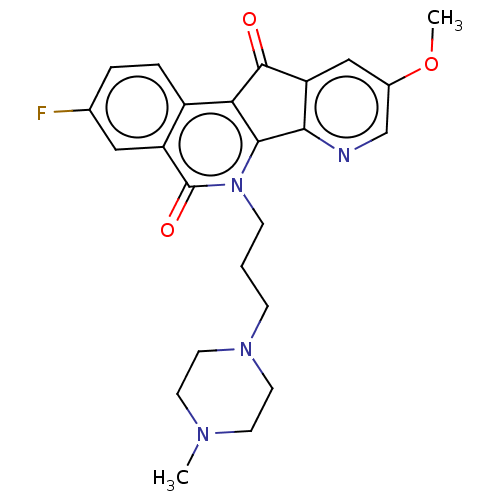 (CHEMBL4068761) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257125 (CHEMBL4094373) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257120 (CHEMBL4072940) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257107 (CHEMBL4079315) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257106 (CHEMBL4077196) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257105 (CHEMBL4064072) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257102 (CHEMBL4090922) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257100 (CHEMBL4079995) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50257121 (CHEMBL4068761) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant TDP1 (unknown origin) using 5'-[32P]-labeled single stranded DNA containing a 3'-phosphotyrosine as substrate after 15 mins... | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257122 (CHEMBL4099136) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50257090 (CHEMBL4072994) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Molecular Pharmacology, College of Pharmacy, and the Purdue Center for Cancer Research, Purdue University , West Lafayette, Indiana 47907, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human TDP2 using 3'-labeled alpha-[32P]-cordycepin as substrate after 15 mins | J Med Chem 60: 5364-5376 (2017) Article DOI: 10.1021/acs.jmedchem.6b01870 BindingDB Entry DOI: 10.7270/Q290266B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-8 (Homo sapiens (Human)) | BDBM50533579 (CHEMBL4519227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-8 N-terminal after 5 mins in presence of fluorescent probe 2-(fluorescein-5-yl-carbonylami... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-9 (Homo sapiens (Human)) | BDBM50533579 (CHEMBL4519227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-9 N-terminal after 5 mins in presence of fluorescent probe 2-(fluorescein-5-yl-carbonylami... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-3 (Homo sapiens (Human)) | BDBM50533580 (CHEMBL4446622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-3 R144K mutant after 5 mins in presence of fluorescent probe 2-(fluorescein-5/6-yl-carbony... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-3 (Homo sapiens (Human)) | BDBM50077225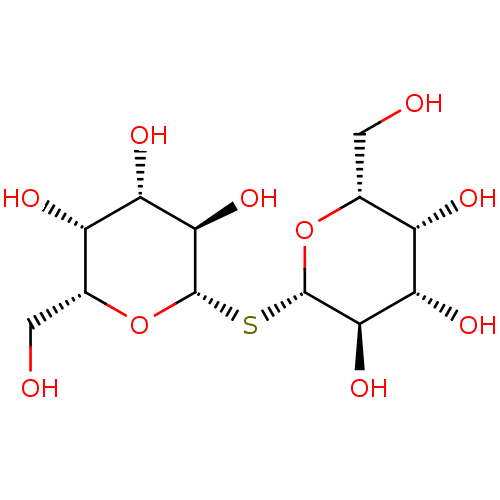 ((2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2S,3R,4S,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to human galectin-3 after 5 mins in presence of fluorescent probe by fluorescence polarization assay | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Galectin-4 (Homo sapiens (Human)) | BDBM50077225 ((2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2S,3R,4S,5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-4 N-terminal after 5 mins in presence of fluorescent probe 3,3'-dideoxy-3-[4-(fluorescein-... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-9 (Homo sapiens (Human)) | BDBM50533581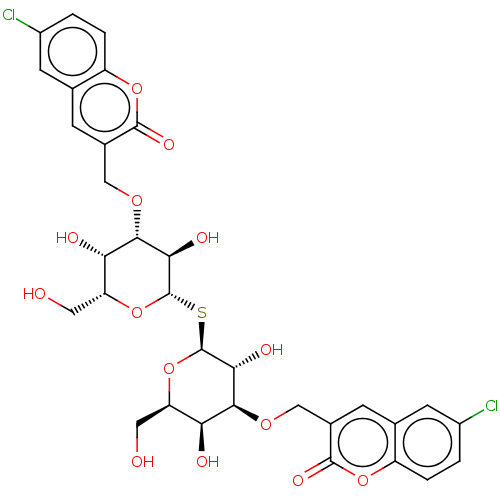 (CHEMBL4588244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-9 N-terminal after 5 mins in presence of fluorescent probe 2-(fluorescein-5-yl-carbonylami... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-1 (Homo sapiens (Human)) | BDBM50533579 (CHEMBL4519227) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-1 after 5 mins in presence of fluorescent probe 3,3'-dideoxy-3-[4-(fluorescein-5-yl-carbon... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-7 (Mus musculus) | BDBM50533581 (CHEMBL4588244) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant mouse galectin-7 after 5 mins in presence of fluorescent probe beta-D-galactopyranosyl(1-4)-2- acetamido-... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-9 (Homo sapiens (Human)) | BDBM50273581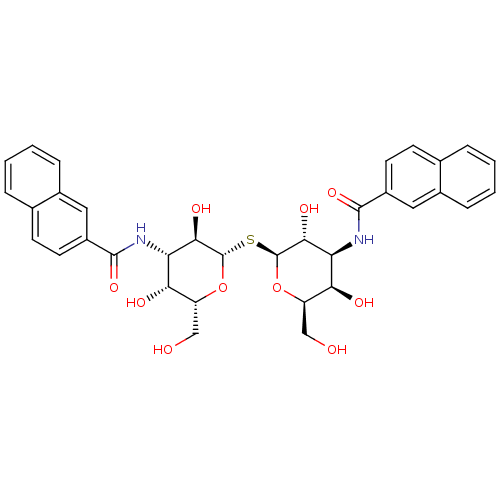 (CHEMBL444662 | N-[(2S,3R,4S,5R,6R)-2-{[(2S,3R,4S,5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-9 N-terminal after 5 mins in presence of fluorescent probe 2-(fluorescein-5-yl-carbonylami... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galectin-3 (Homo sapiens (Human)) | BDBM50533580 (CHEMBL4446622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a |
Institute of Science Education and Research-Kolkata (IISER) Kolkata Curated by ChEMBL | Assay Description Competitive binding affinity to recombinant human galectin-3 R144S mutant after 5 mins in presence of fluorescent probe 2-(fluorescein-5/6-yl-carbony... | J Med Chem 59: 8141-7 (2016) Article DOI: 10.1021/acs.jmedchem.6b00957 BindingDB Entry DOI: 10.7270/Q2X63RF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |