

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50113342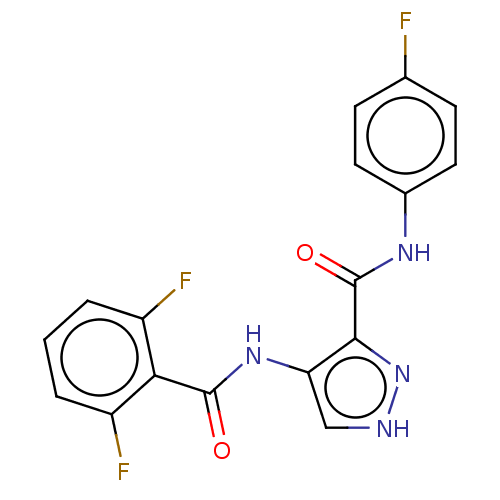 (CHEMBL518383) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem | DrugBank PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368878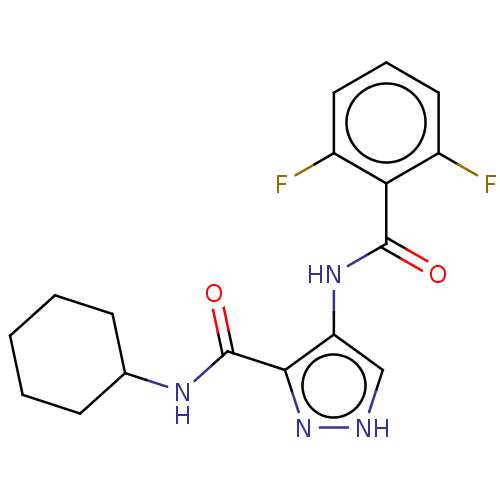 (CHEMBL456963) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368899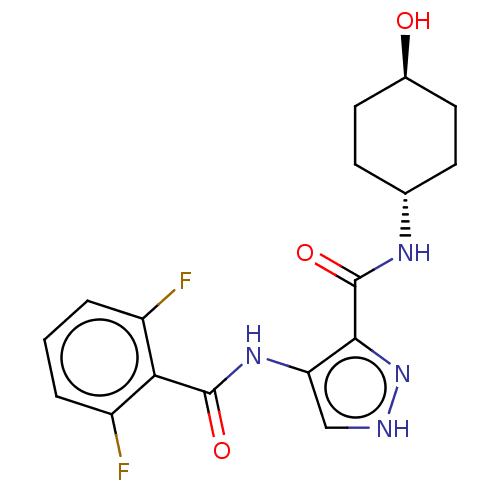 (CHEMBL457177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368881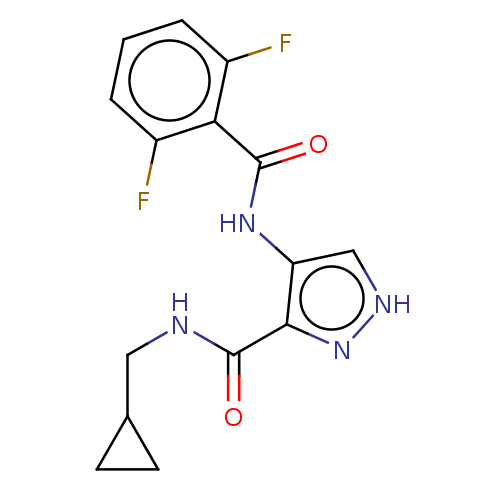 (CHEMBL510578) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368879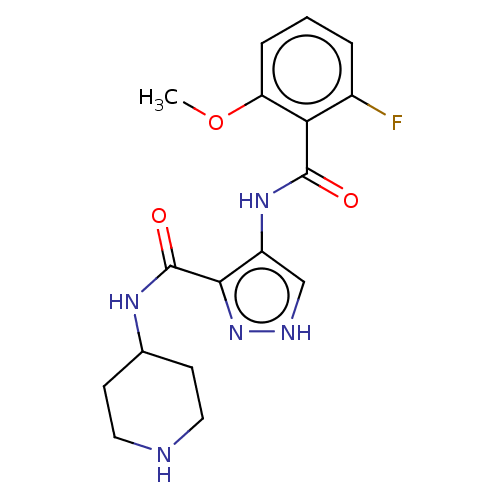 (CHEMBL497306) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins in presence of NADPH | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368876 (CHEMBL507167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368900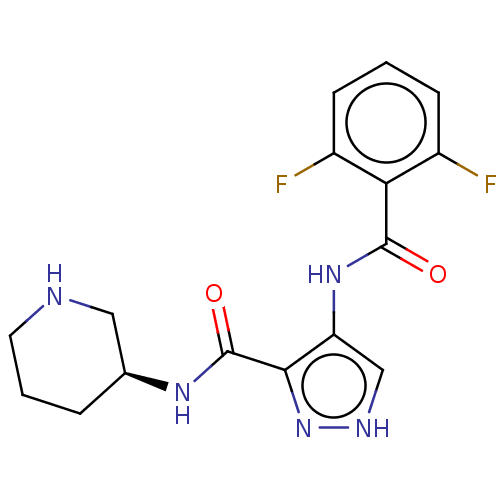 (CHEMBL1187319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50113281 (AT-7519) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517400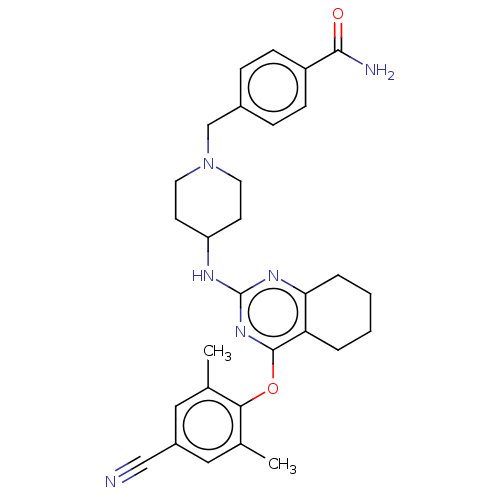 (CHEMBL4525390) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517387 (CHEMBL4514678) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517395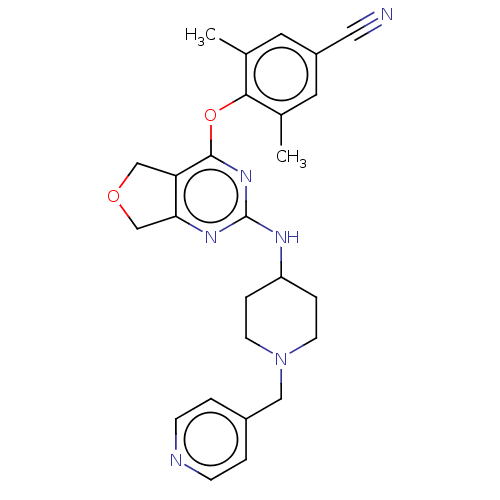 (CHEMBL4570398) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517407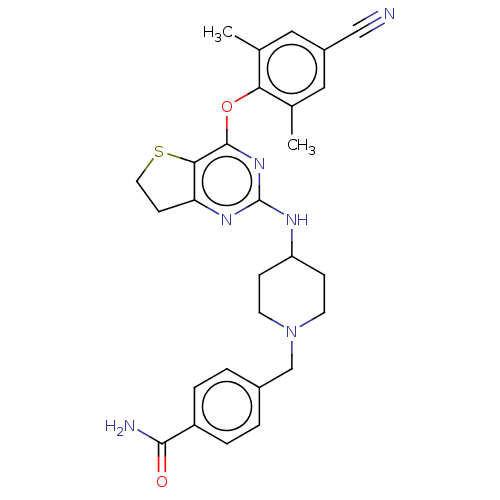 (CHEMBL4594064) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517388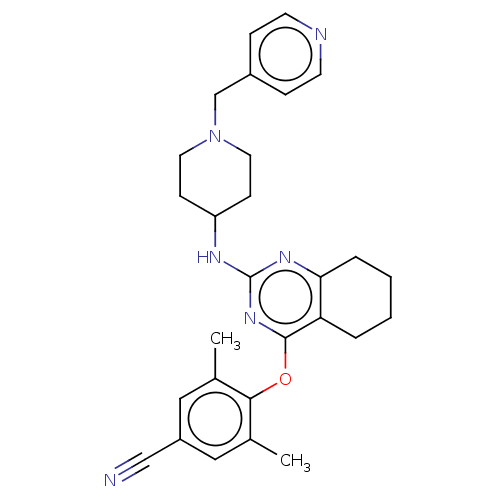 (CHEMBL4454996) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517410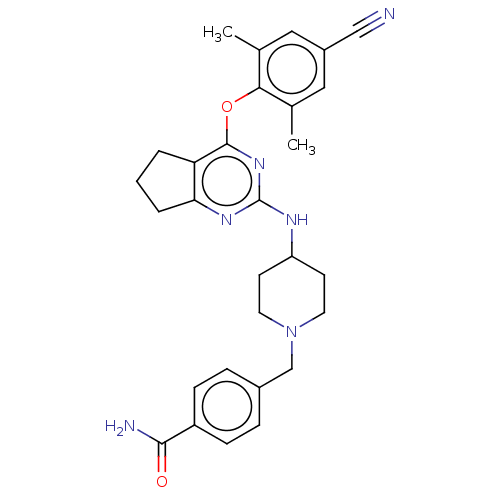 (CHEMBL4464437) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517397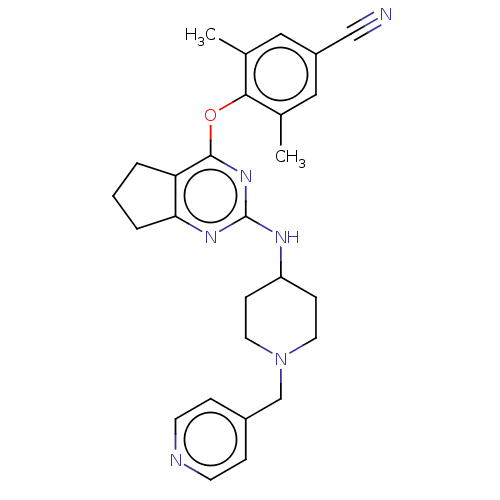 (CHEMBL4563462) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517389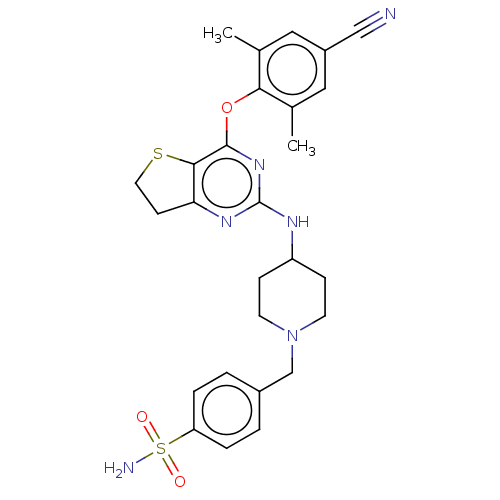 (CHEMBL4474372) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517409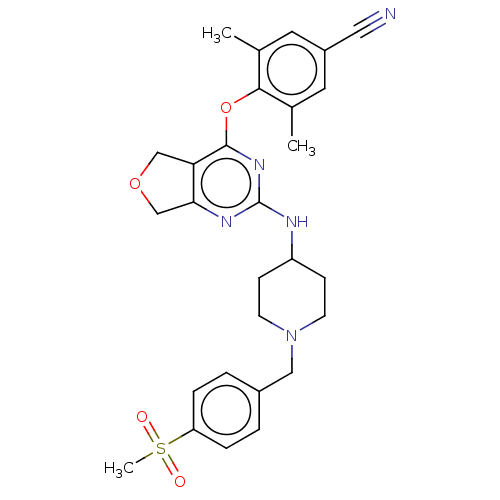 (CHEMBL4557661) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517411 (CHEMBL4451654) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50017376 ((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by manual patch clamp assay | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517401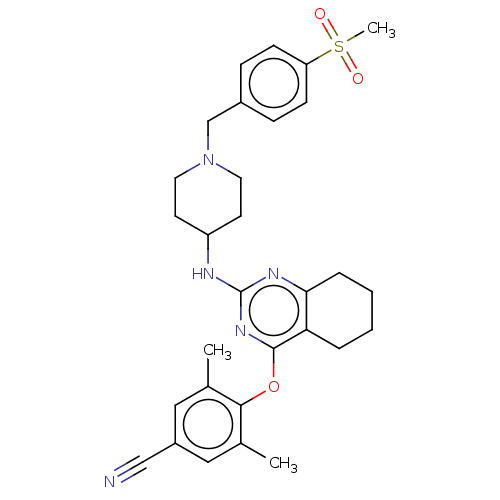 (CHEMBL4577248) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368874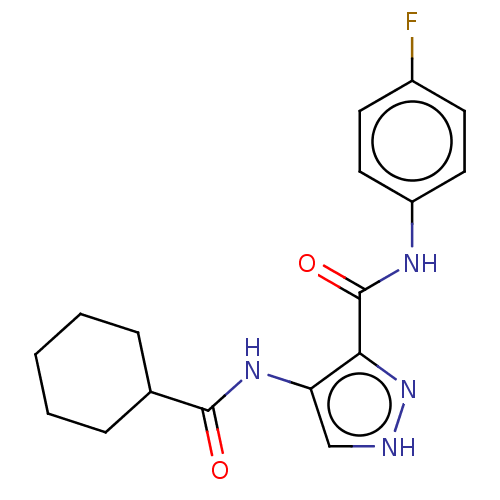 (CHEMBL463579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517385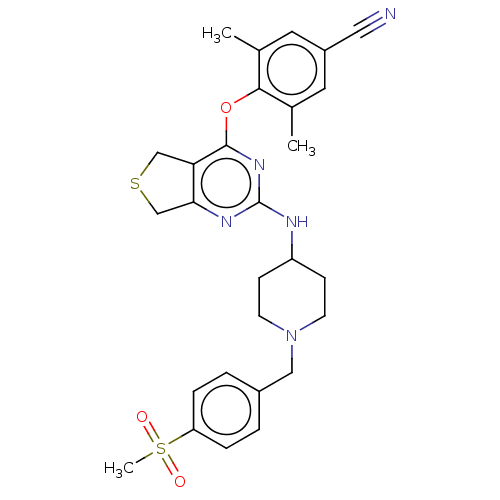 (CHEMBL4570178) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517406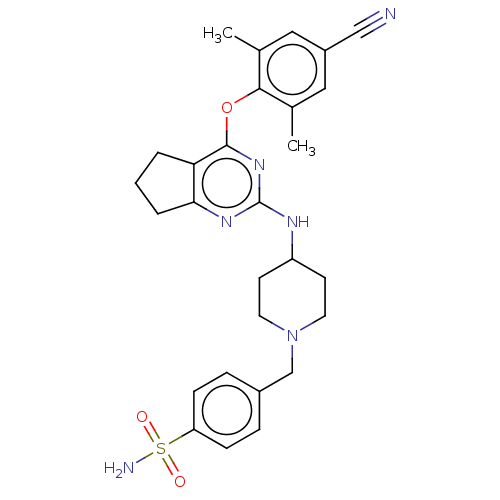 (CHEMBL4561250) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517386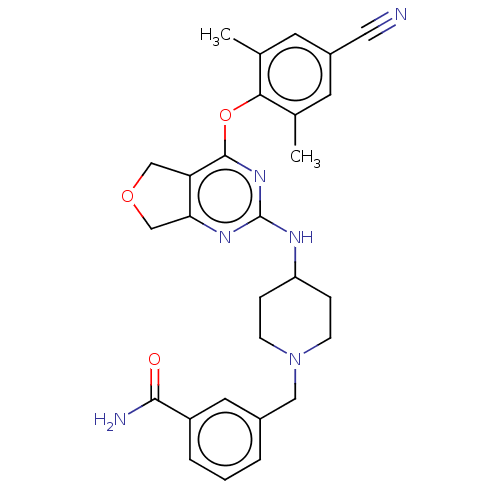 (CHEMBL4563214) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517396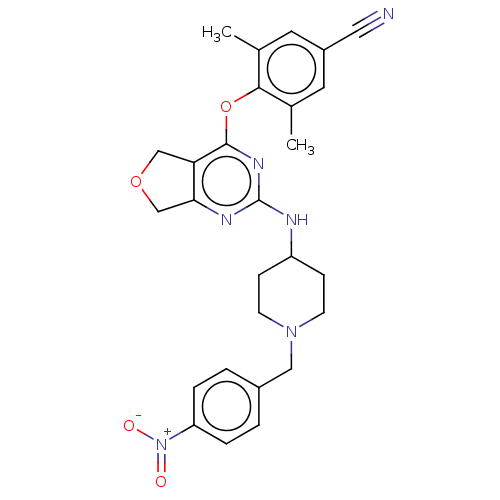 (CHEMBL4586013) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517391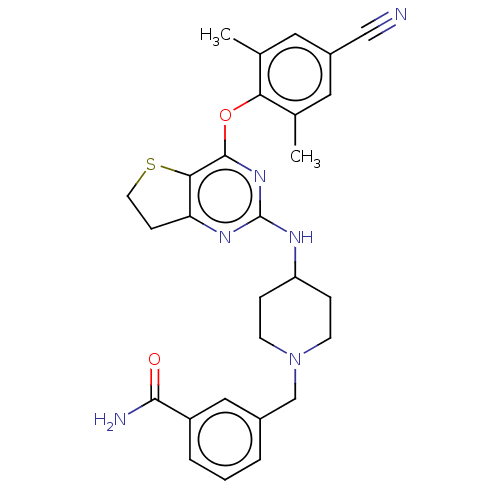 (CHEMBL4533242) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517413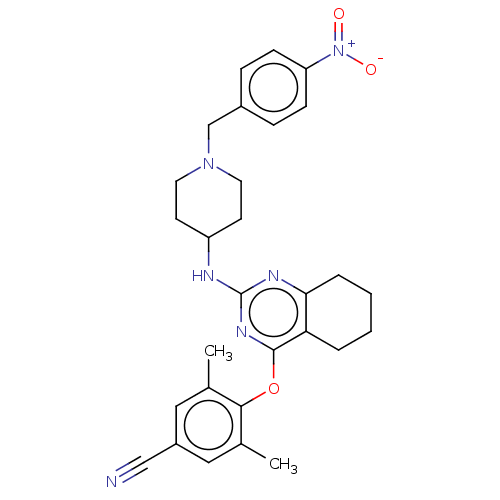 (CHEMBL4553824) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517412 (CHEMBL4545315) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517403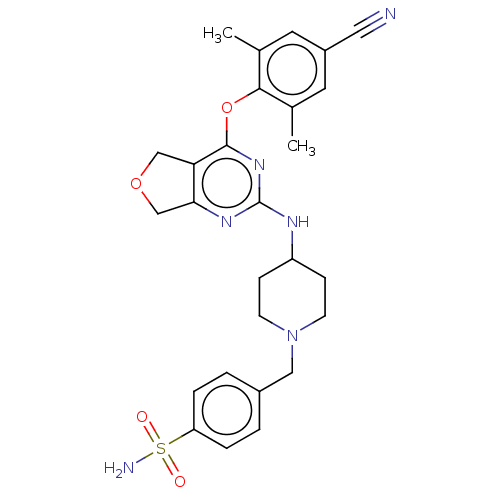 (CHEMBL4437991) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368902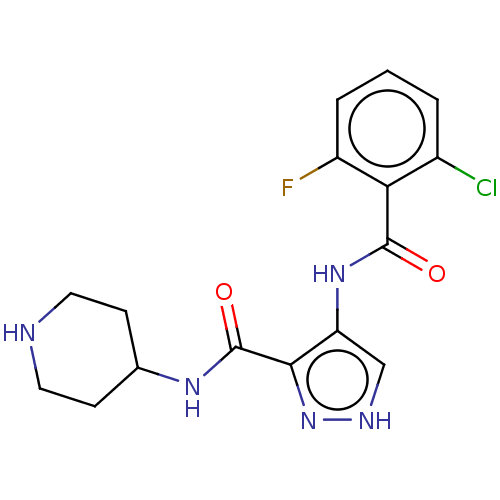 (CHEMBL497477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517390 (CHEMBL4570889) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517393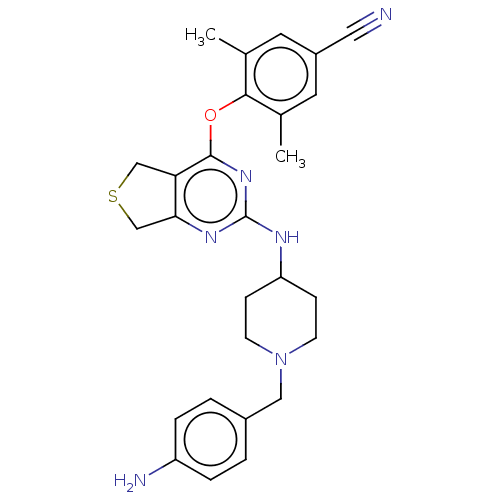 (CHEMBL4469840) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517408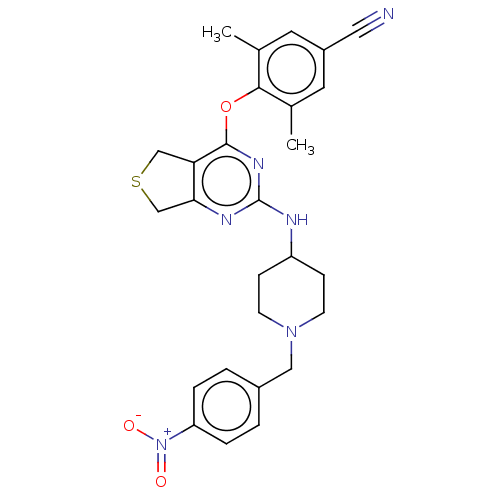 (CHEMBL4546225) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517405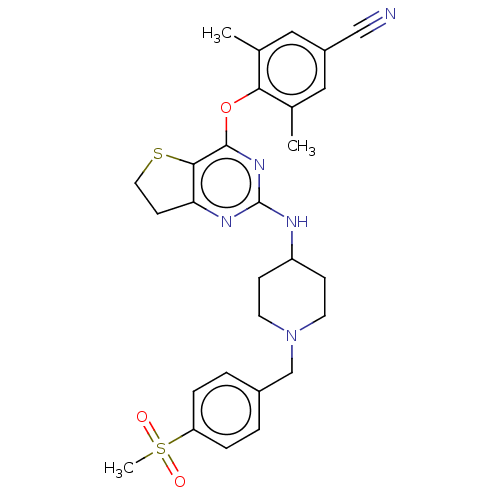 (CHEMBL4553083) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50113349 (CHEMBL457388) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517394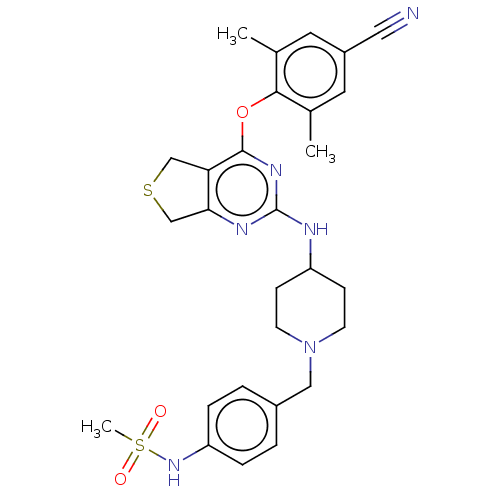 (CHEMBL4445956) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368897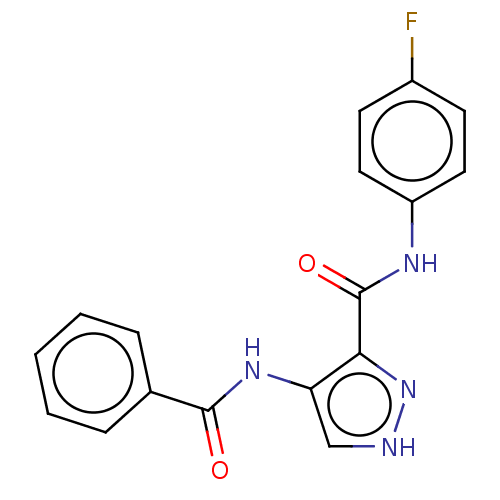 (CHEMBL463580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 mins in presence of NADPH | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517404 (CHEMBL4464124) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517402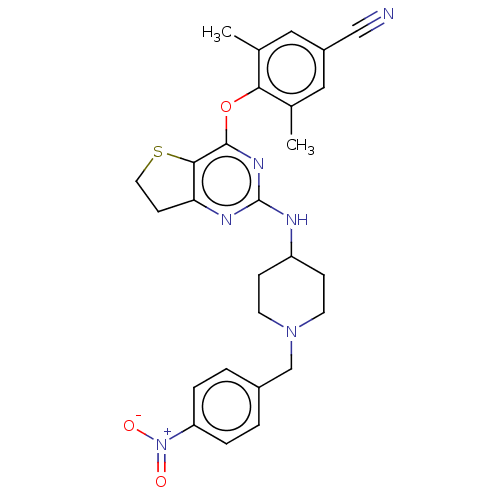 (CHEMBL4526807) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins in presence of NADPH | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517398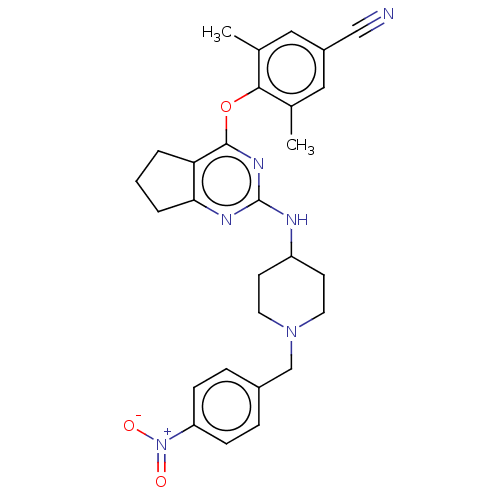 (CHEMBL4442432) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517399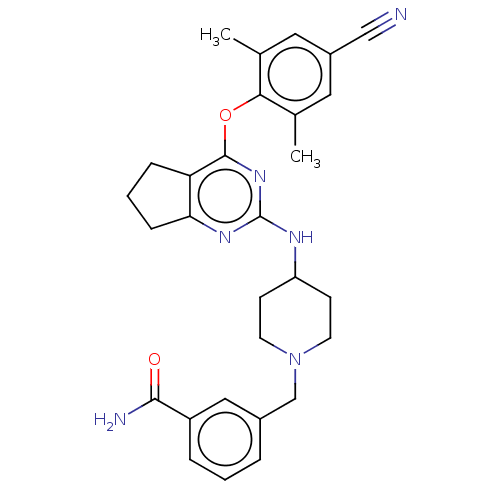 (CHEMBL4436487) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50207551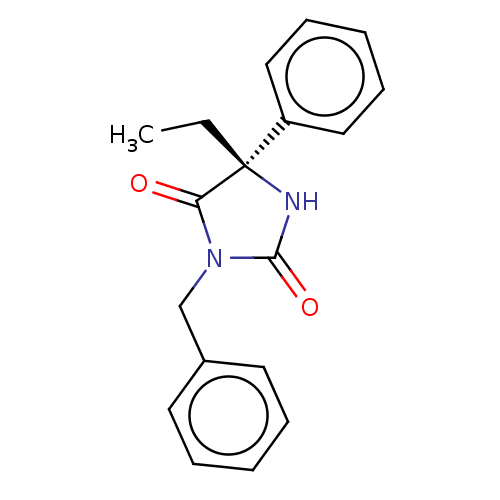 (CHEMBL3977345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 10 mins in presence of NADPH | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50517392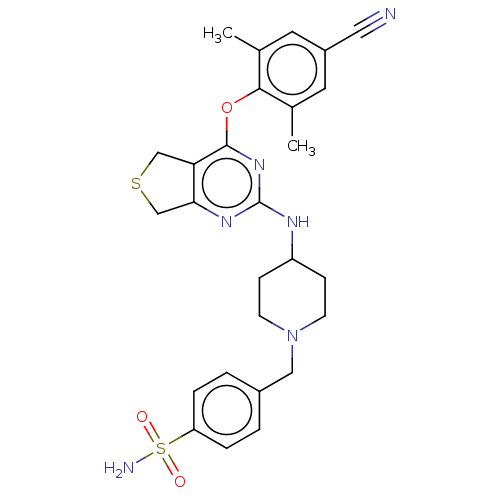 (CHEMBL4584556) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase using (DIG)-dUTP and biotin-labeled dNTPs as substrate after 1 hr by ELISA | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677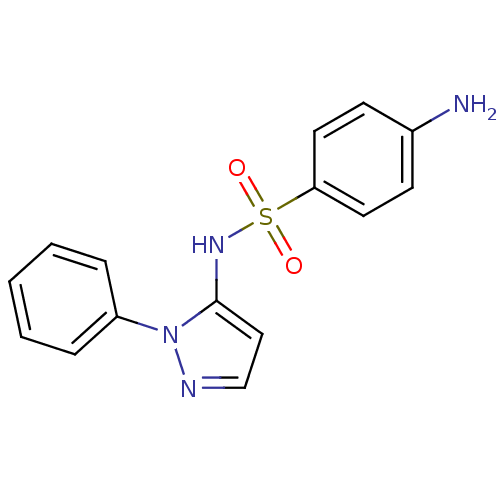 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 10 mins in presence of NADPH | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50368905 (CHEMBL448316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human full length CDK2/N-terminal GST-tagged human full length Cyclin A using histone H1 and [gamma-32P]-ATP inc... | J Med Chem 61: 7700-7709 (2018) Article DOI: 10.1021/acs.jmedchem.8b00669 BindingDB Entry DOI: 10.7270/Q2GX4F3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50517387 (CHEMBL4514678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by manual patch clamp assay | J Med Chem 62: 1484-1501 (2019) Article DOI: 10.1021/acs.jmedchem.8b01656 BindingDB Entry DOI: 10.7270/Q29P351Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |