

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50516168 (CHEMBL4541269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50516168 (CHEMBL4541269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50516168 (CHEMBL4541269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50516168 (CHEMBL4541269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50516168 (CHEMBL4541269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50326793 ((+/-)(1S,2S,3R)-2-[(S)-Amino(carboxy)methyl]-3-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Antagonist activity at mGlu2 receptor expressed in CHO cells assessed as increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50326793 ((+/-)(1S,2S,3R)-2-[(S)-Amino(carboxy)methyl]-3-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516168 (CHEMBL4541269) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Positive allosteric modulatory activity at rat alpha7 nAChR expressed in GH4C1 cells assessed as potentiation of acetylcholine response at -70 mV hol... | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516169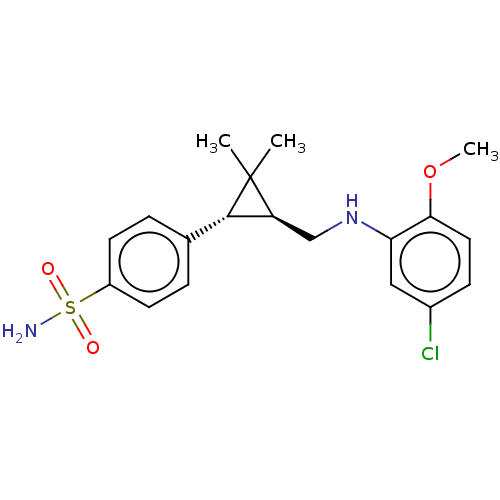 (CHEMBL4435397) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Positive allosteric modulatory activity at rat alpha7 nAChR expressed in GH4C1 cells assessed as potentiation of acetylcholine response at -70 mV hol... | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50516170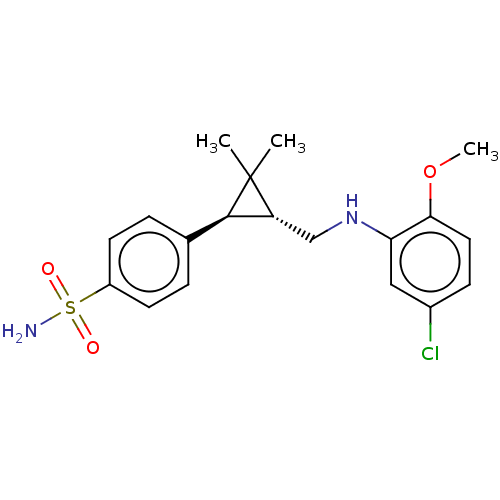 (CHEMBL4456125) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Bionomics Limited Curated by ChEMBL | Assay Description Positive allosteric modulatory activity at rat alpha7 nAChR expressed in GH4C1 cells assessed as potentiation of acetylcholine response at -70 mV hol... | ACS Med Chem Lett 10: 754-760 (2019) Article DOI: 10.1021/acsmedchemlett.9b00001 BindingDB Entry DOI: 10.7270/Q25D8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50326794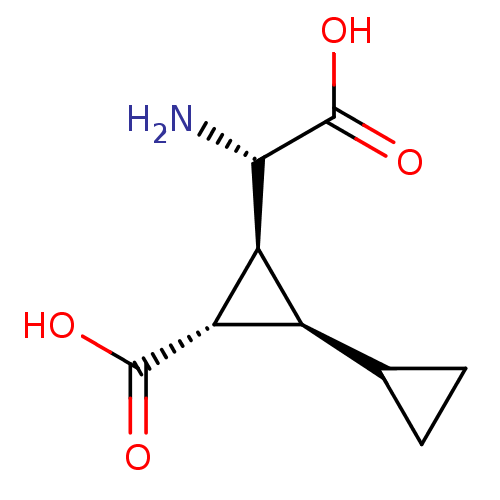 ((+/-)(1R,2S,3S)-3-[(S)-amino(carboxy)-methyl]-1,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM50138781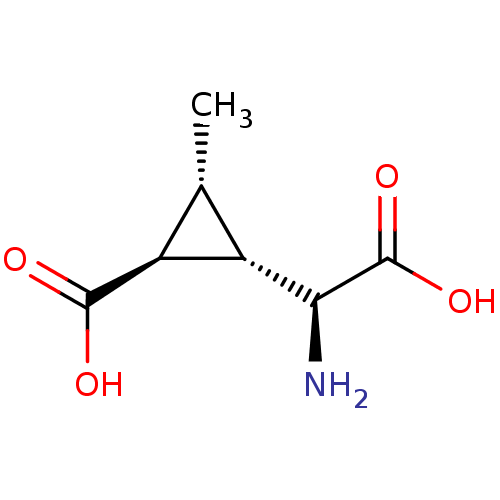 ((1S,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-methyl-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50326794 ((+/-)(1R,2S,3S)-3-[(S)-amino(carboxy)-methyl]-1,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >50 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50138781 ((1S,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-methyl-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >10 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP level | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50138781 ((1S,2S,3R)-2-((S)-Amino-carboxy-methyl)-3-methyl-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation counting | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50326794 ((+/-)(1R,2S,3S)-3-[(S)-amino(carboxy)-methyl]-1,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation counting | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50326793 ((+/-)(1S,2S,3R)-2-[(S)-Amino(carboxy)methyl]-3-cyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation counting | Bioorg Med Chem 18: 6089-98 (2010) Article DOI: 10.1016/j.bmc.2010.06.051 BindingDB Entry DOI: 10.7270/Q2C24WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||