

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Selectivity against alphaV-beta3 integrin | J Nat Prod 80: 2818-2824 (2017) Article DOI: 10.1021/acs.jnatprod.7b00677 BindingDB Entry DOI: 10.7270/Q2571FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Bioactive Natural Material Bank, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate after 30 mins | Bioorg Med Chem Lett 27: 5076-5081 (2017) Article DOI: 10.1016/j.bmcl.2017.09.031 BindingDB Entry DOI: 10.7270/Q2C24ZXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50262424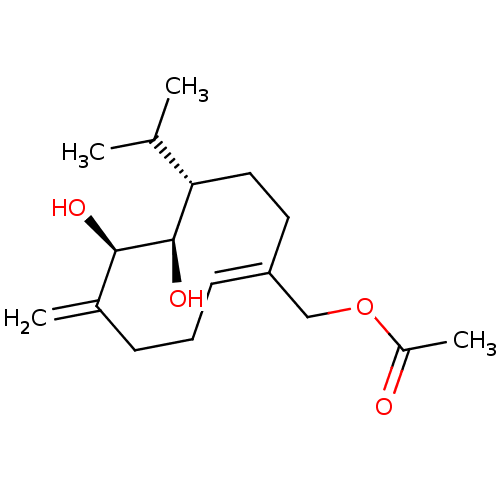 (CHEMBL4105170) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Bioactive Natural Material Bank, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate after 30 mins | Bioorg Med Chem Lett 27: 5076-5081 (2017) Article DOI: 10.1016/j.bmcl.2017.09.031 BindingDB Entry DOI: 10.7270/Q2C24ZXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50262425 (CHEMBL4104518) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Bioactive Natural Material Bank, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate after 30 mins | Bioorg Med Chem Lett 27: 5076-5081 (2017) Article DOI: 10.1016/j.bmcl.2017.09.031 BindingDB Entry DOI: 10.7270/Q2C24ZXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50262426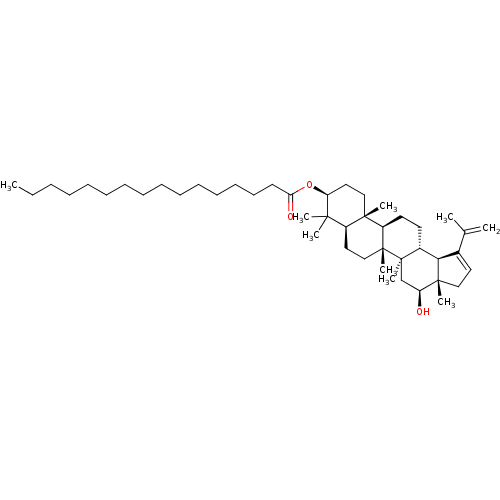 (CHEMBL4067572) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Bioactive Natural Material Bank, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate after 30 mins | Bioorg Med Chem Lett 27: 5076-5081 (2017) Article DOI: 10.1016/j.bmcl.2017.09.031 BindingDB Entry DOI: 10.7270/Q2C24ZXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50278271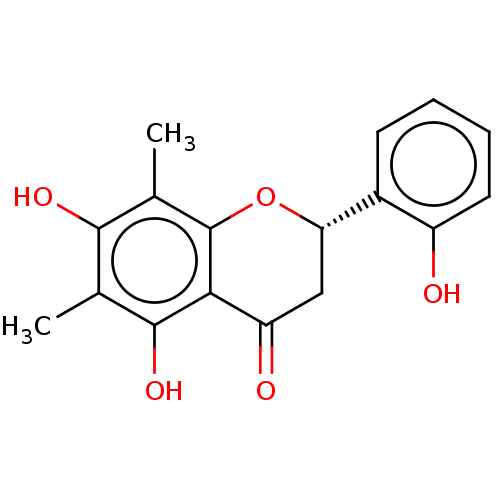 ((2S)-2''-Hydroxydemethoxymatteucinol | CHEBI:67371...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity in Influenza A virus (A/PR/8/34 (H1N1)) infected in MDCK cells using 4-MU-NANA as substrate pretreated for 30 mi... | J Nat Prod 80: 2818-2824 (2017) Article DOI: 10.1021/acs.jnatprod.7b00677 BindingDB Entry DOI: 10.7270/Q2571FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50278268 (CHEMBL4168939) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity in Influenza A virus (A/PR/8/34 (H1N1)) infected in MDCK cells using 4-MU-NANA as substrate pretreated for 30 mi... | J Nat Prod 80: 2818-2824 (2017) Article DOI: 10.1021/acs.jnatprod.7b00677 BindingDB Entry DOI: 10.7270/Q2571FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50278270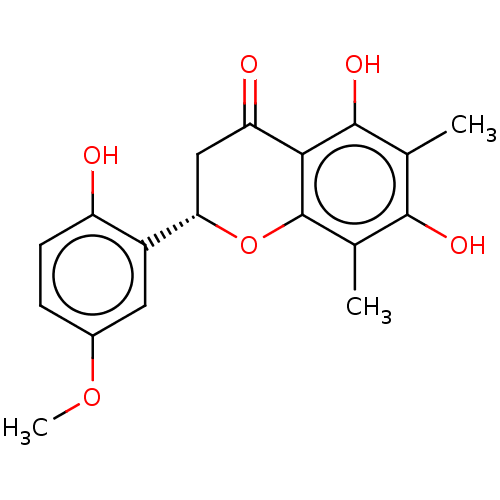 (CHEMBL4161011) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity in Influenza A virus (A/PR/8/34 (H1N1)) infected in MDCK cells using 4-MU-NANA as substrate pretreated for 30 mi... | J Nat Prod 80: 2818-2824 (2017) Article DOI: 10.1021/acs.jnatprod.7b00677 BindingDB Entry DOI: 10.7270/Q2571FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50278272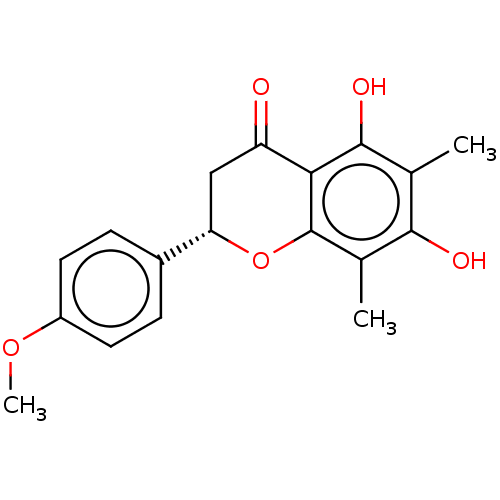 (CHEMBL4164421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of neuraminidase activity in Influenza A virus (A/PR/8/34 (H1N1)) infected in MDCK cells using 4-MU-NANA as substrate pretreated for 30 mi... | J Nat Prod 80: 2818-2824 (2017) Article DOI: 10.1021/acs.jnatprod.7b00677 BindingDB Entry DOI: 10.7270/Q2571FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50262423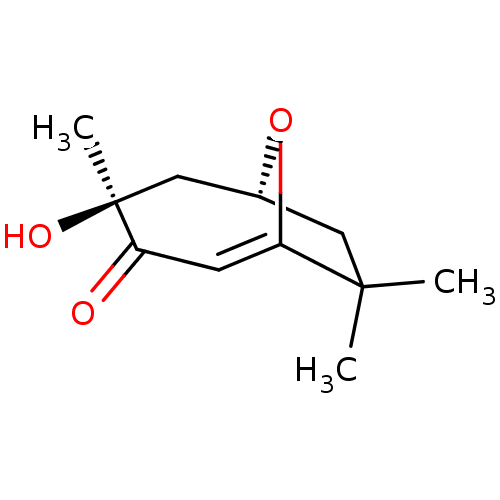 (CHEMBL4087311) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Bioactive Natural Material Bank, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using pNPP as substrate after 30 mins | Bioorg Med Chem Lett 27: 5076-5081 (2017) Article DOI: 10.1016/j.bmcl.2017.09.031 BindingDB Entry DOI: 10.7270/Q2C24ZXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50278269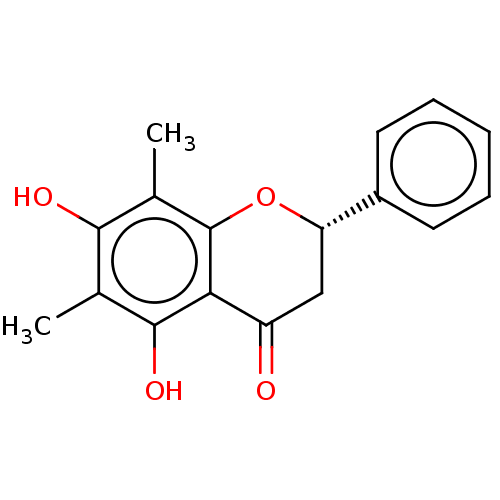 (6,8-Dimethylpinocembrin | CHEBI:70664 | Demethoxym...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibitory activity against alphaV-beta3 integrin | J Nat Prod 80: 2818-2824 (2017) Article DOI: 10.1021/acs.jnatprod.7b00677 BindingDB Entry DOI: 10.7270/Q2571FJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||