

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pantetheinase (Homo sapiens (Human)) | BDBM408834 (US10364255, Ex. 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184269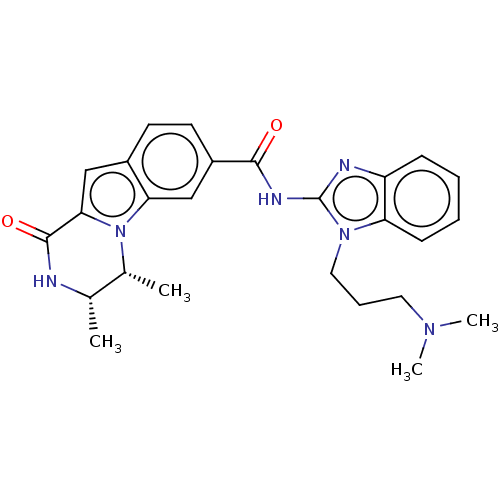 (US9150577, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408835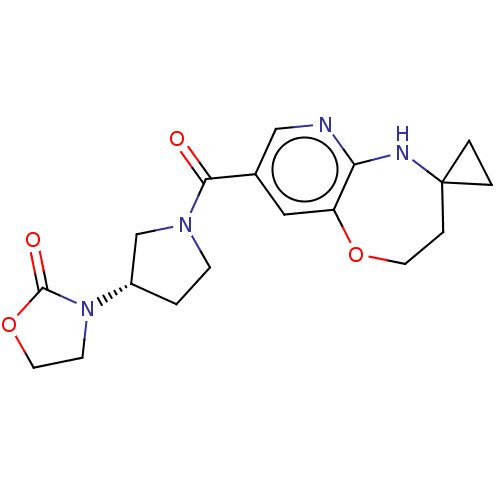 (US10364255, Ex. 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM168025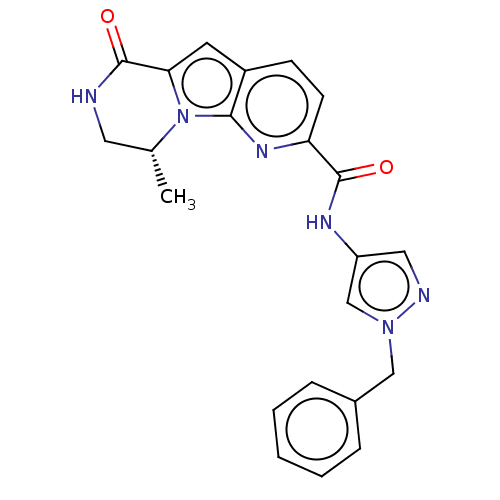 (US9073926, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9073926 (2015) BindingDB Entry DOI: 10.7270/Q2BP01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408845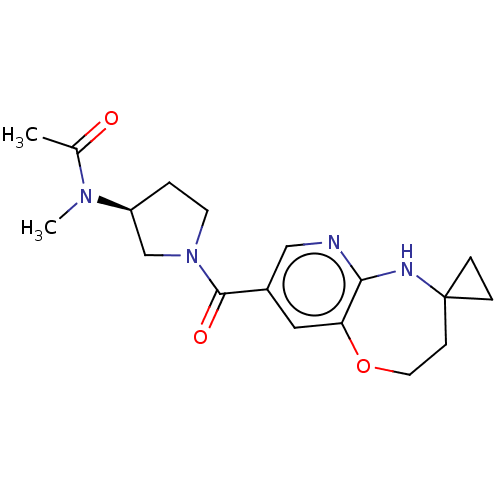 (US10364255, Ex. 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289984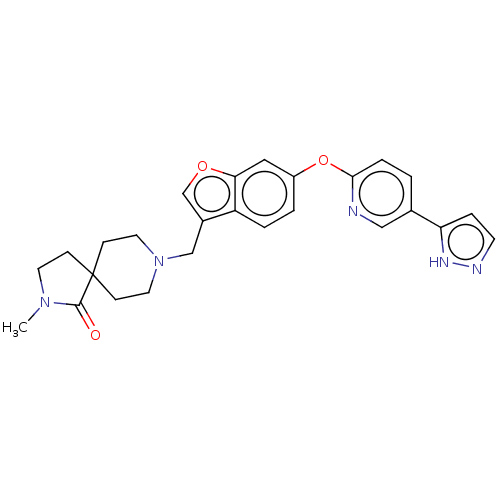 (2-Methyl-8-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-ylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184222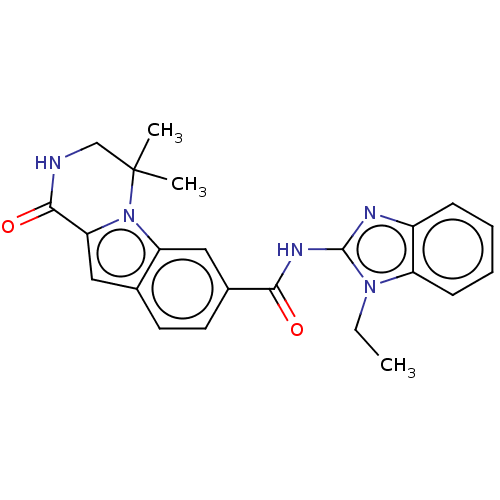 (US9150577, 82) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM168034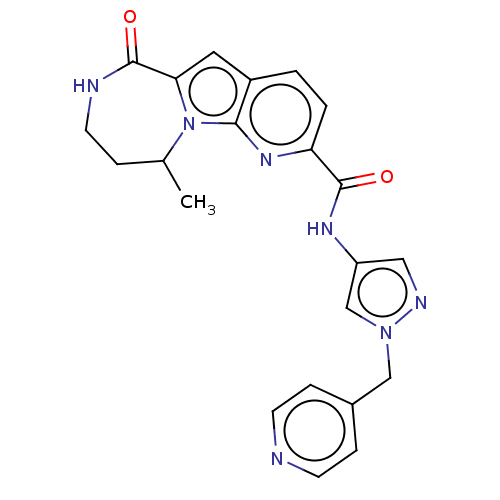 (US9073926, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9073926 (2015) BindingDB Entry DOI: 10.7270/Q2BP01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289920 (2-Methoxy-1-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289869 (N-((R)-1-{6-[5-(2H-Pyrazol-3-yl)- pyridin-2-yloxy]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM168027 (US9073926, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9073926 (2015) BindingDB Entry DOI: 10.7270/Q2BP01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289983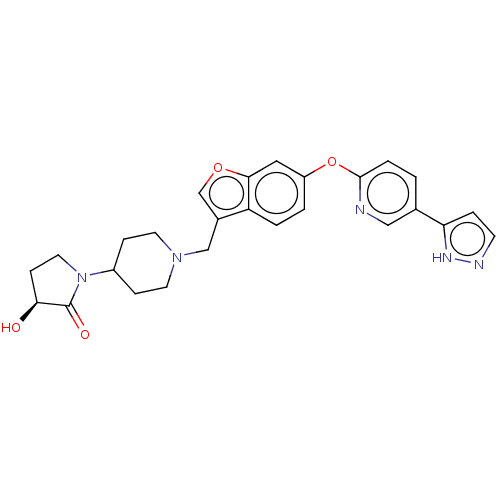 ((S)-3-Hydroxy-1-(1-{6-[5-(2H- pyrazol-3-yl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184235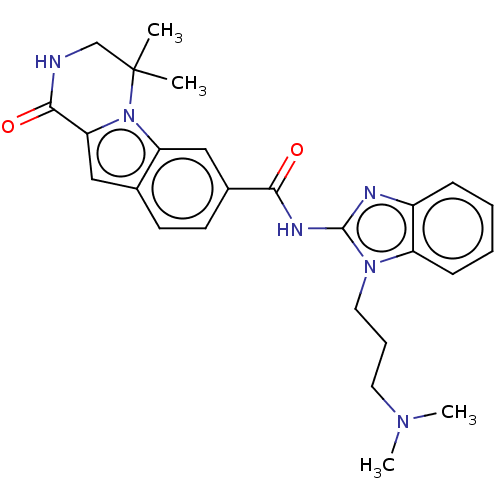 (US9150577, 98) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289980 (N-(1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM168012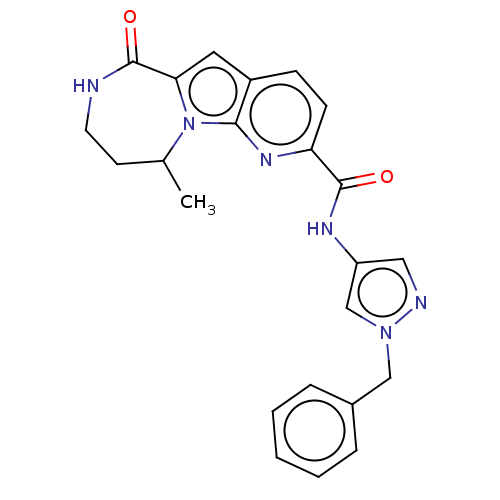 (US9073926, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9073926 (2015) BindingDB Entry DOI: 10.7270/Q2BP01J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408829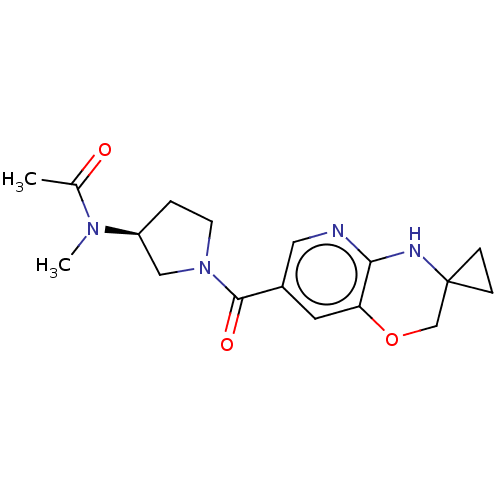 (US10364255, Ex. 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289864 (2-Hydroxy-1-(4-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408828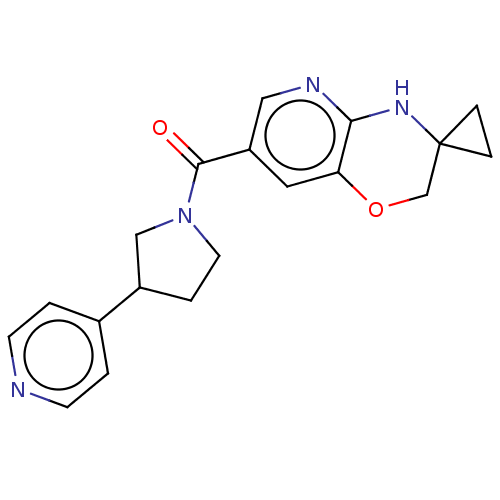 (US10364255, Ex. 20 | US10364255, Ex. 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184270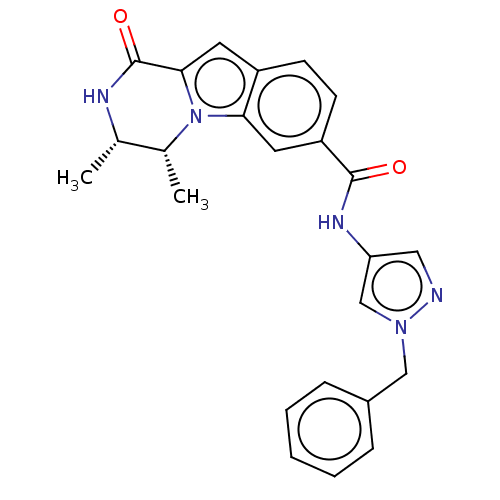 (US9150577, 140) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289987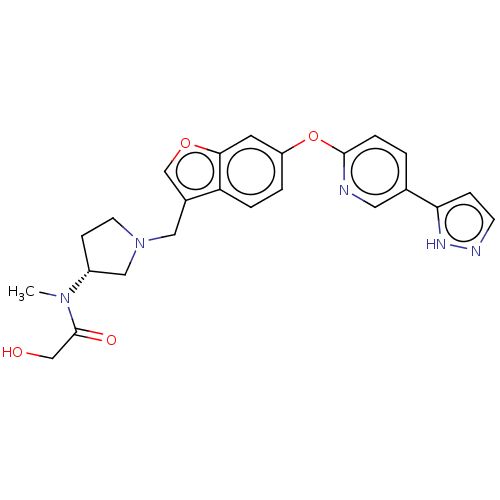 (2-Hydroxy-N-methyl-N-((R)-1-{6- [5-(2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289989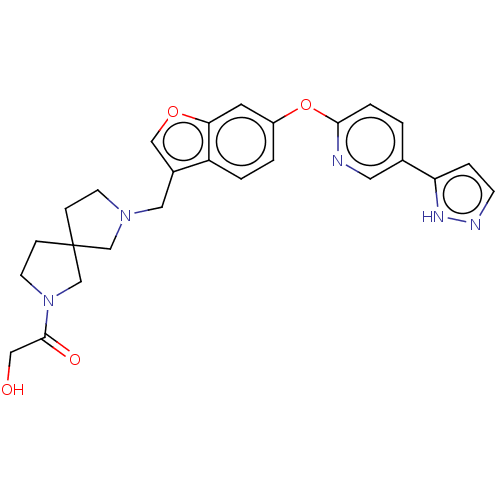 (2-Hydroxy-1-(7-{6-[5-(2H-pyrazol- 3-yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289917 ((S)-2-Hydroxy-1-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289897 (1-(4-{6-[5-(2H-Pyrazol-3-yl)-pyrimidin- 2-yloxy]-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289858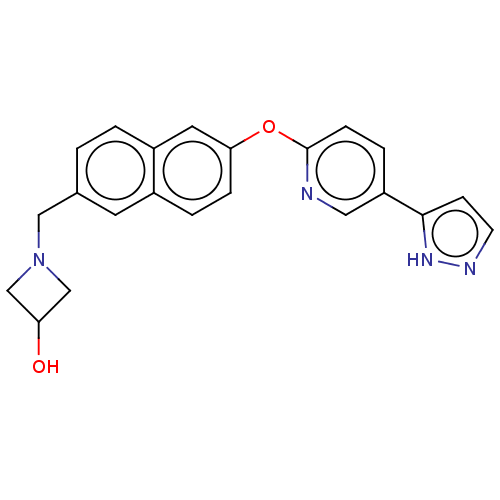 (1-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2-yloxy]-naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289918 (2-Hydroxy-2-methyl-1-(8-{6-[5-(2H- pyrazol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289859 (1-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2- yloxy]-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289932 (1-[1-(2-{6-[5-(2H-Pyrazol-3-yl)- pyridin-2-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM290023 (2-Methyl-3-oxo-3-(4-{6-[5-(2H-pyrazol- 3-yl)-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289867 (2-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2-yloxy]-naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289861 ((1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289976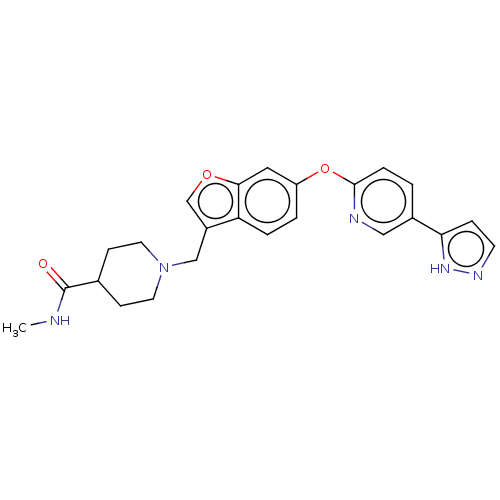 (1-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2- yloxy]-benzof...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408822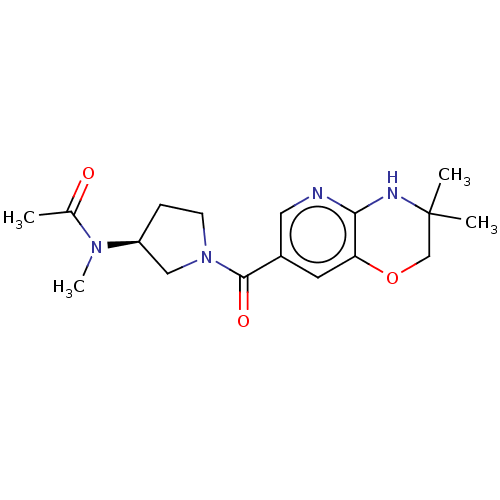 (US10364255, Ex. 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289866 (2-Hydroxy-N-(1-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289860 (N-(1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289916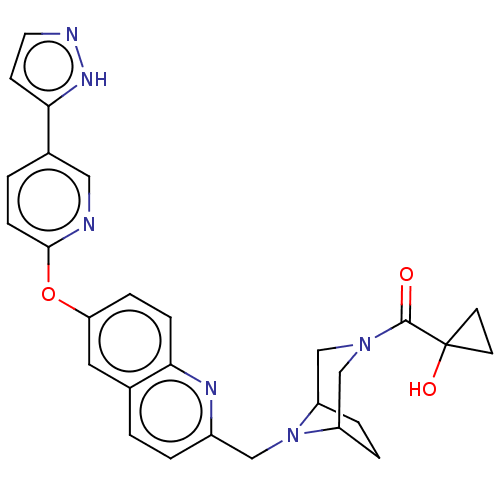 ((1-Hydroxy-cyclopropyl)-(8-{6-[5- (2H-pyrazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289868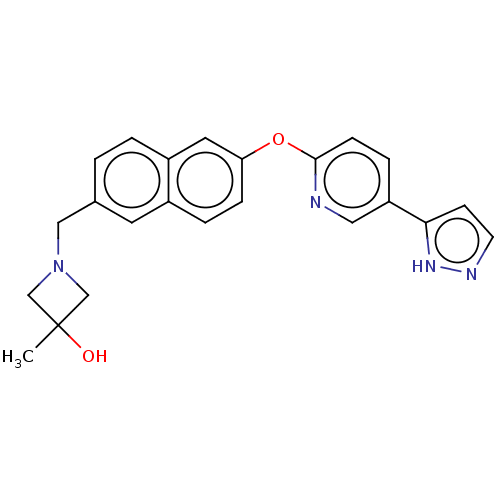 (3-Methyl-1-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-ylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289919 ((R)-2-Hydroxy-1-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289977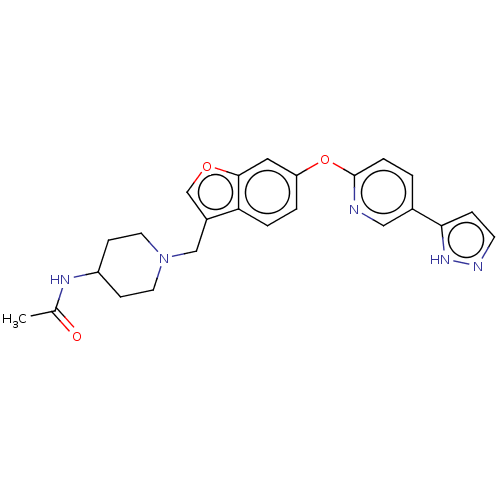 (N-(1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM290002 (2-Methoxy-2-methyl-1-(4-{6- [5-(2H-pyrazol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289988 (2-Hydroxy-N-methyl-N-((S)-1-{6- [5-(2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289863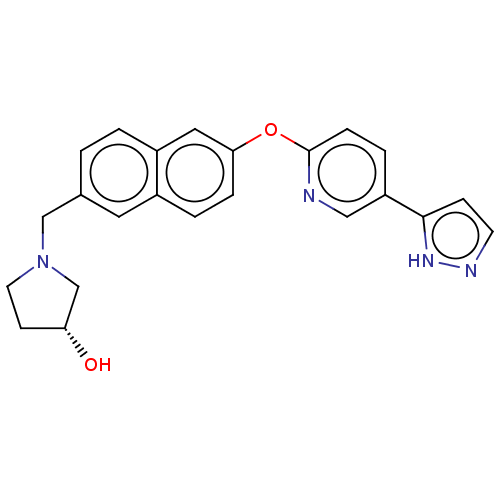 ((R)-1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM290014 ((R)-2-Methoxy-1-(4-{6-[5-(2H-pyrazol- 3-yl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289970 (1-(4-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2-yloxy]-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184201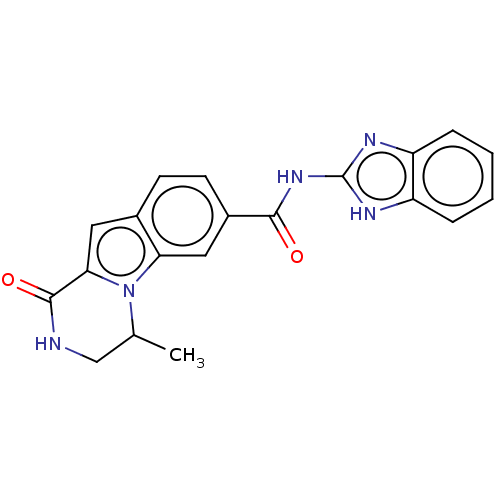 (US9150577, 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289968 (1-(4-{6-[4-(2H-Pyrazol-3-yl)- phenoxy]-imidazo[1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408824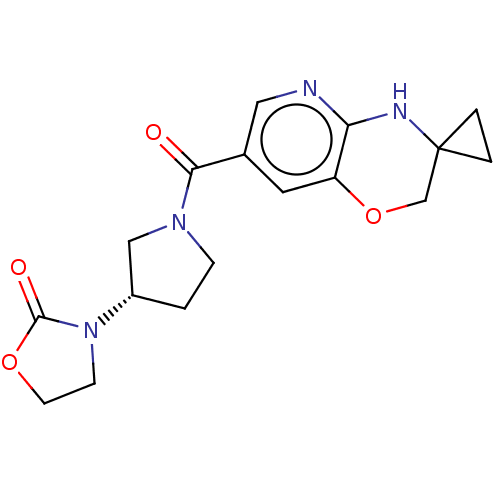 (US10364255, Ex. 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM408833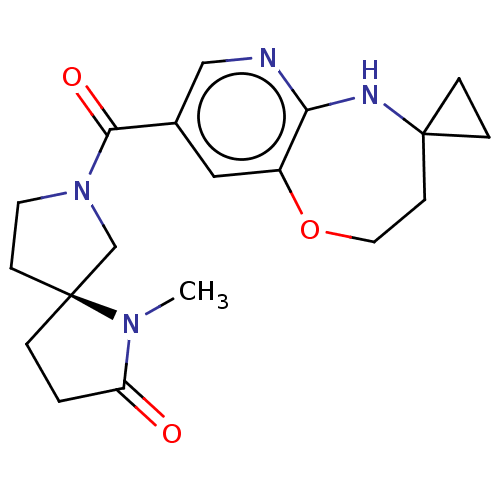 (US10364255, Ex. 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingleheim International GmbH US Patent | Assay Description Method 1:Vanin-1 Enzymatic Assay:The test compounds are dissolved in 100% DMSO at a concentration of 10 mM and in a first step diluted in DMSO to a c... | US Patent US10364255 (2019) BindingDB Entry DOI: 10.7270/Q28S4S8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289862 ((S)-1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184290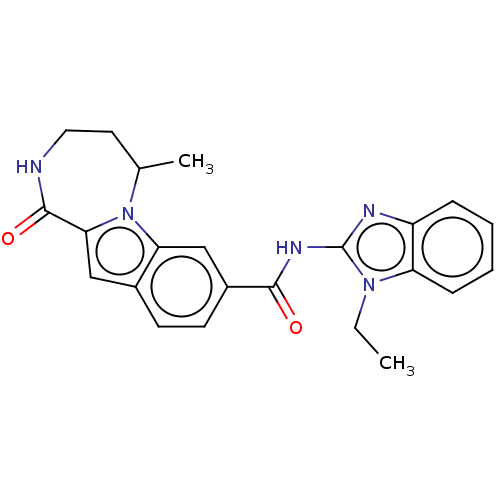 (US9150577, 166) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-2 (Homo sapiens (Human)) | BDBM184260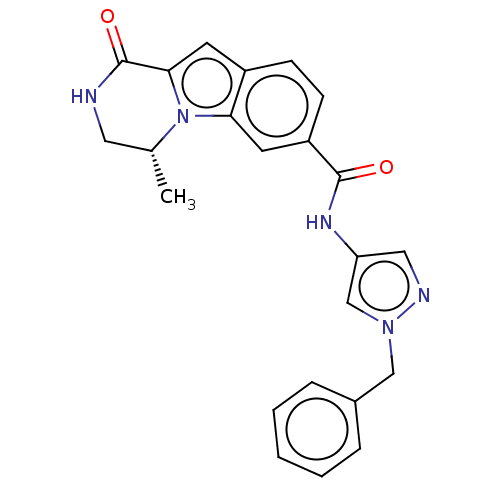 (US9150577, 130) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Human RSK2 protein, purchased from Invitrogen, is used to measure kinase activity utilizing Kinase Glo Plus (Promega) a homogeneous assay technology,... | US Patent US9150577 (2015) BindingDB Entry DOI: 10.7270/Q2GB22V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 598 total ) | Next | Last >> |