

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506203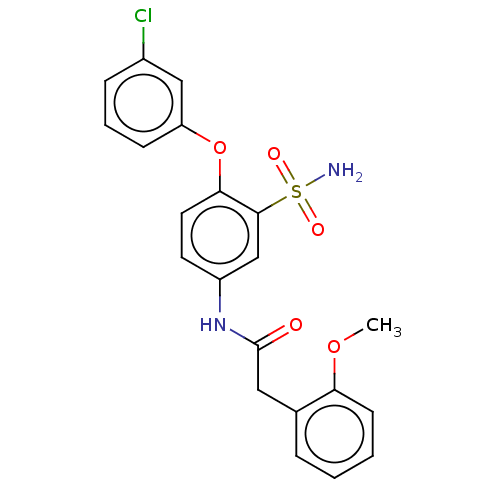 (CHEMBL4452312) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506156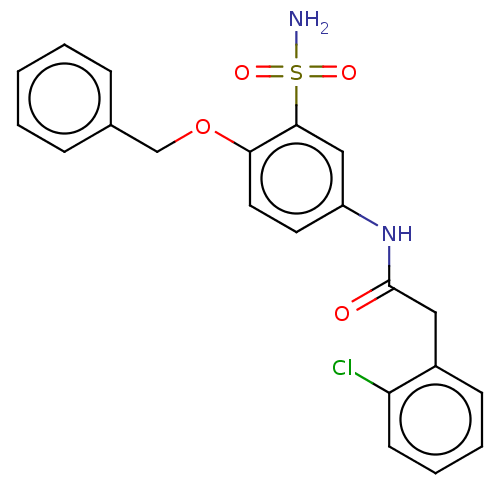 (CHEMBL4471140) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50537001 (CHEMBL4520937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261864 (2-(9-Ethyl-9H-carbazol-3-yl)-4-fluoro-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261893 (2-(6-Chloro-9-ethyl-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50537002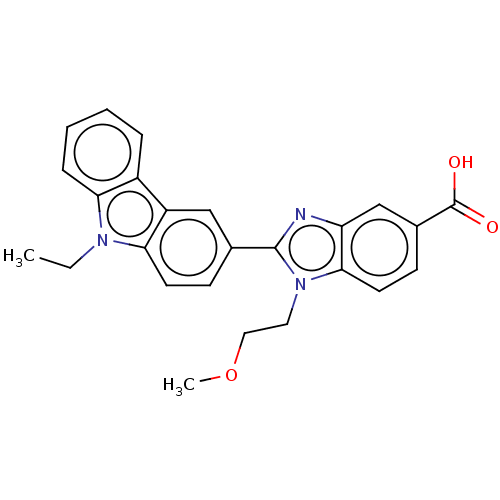 (CHEMBL4483026) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506173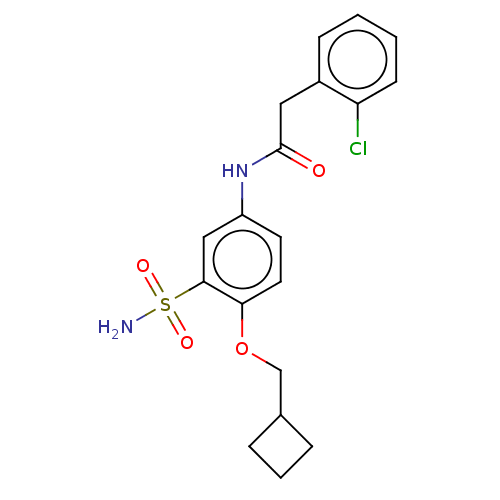 (CHEMBL4589444) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261894 (2-(8-Chloro-9-ethyl-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261795 (3-[2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261794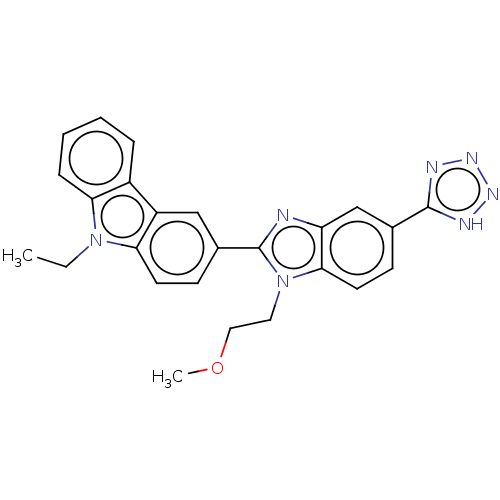 (9-Ethyl-3-[1-(2-methoxyethyl)-5-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261884 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(tetrahydrofuran-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506179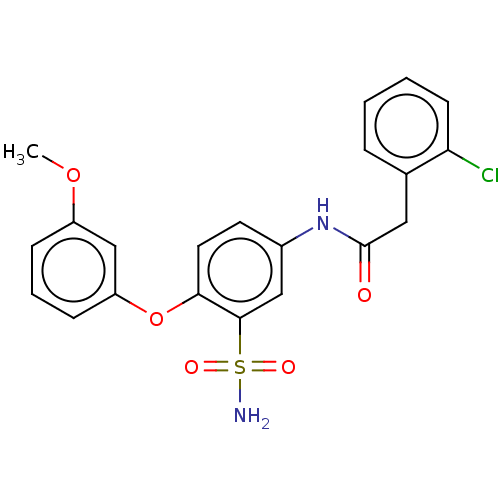 (CHEMBL4579583) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506184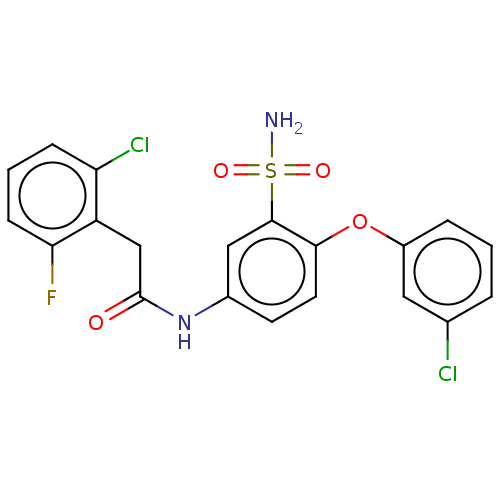 (CHEMBL4447043) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548475 (CHEMBL4747248) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548486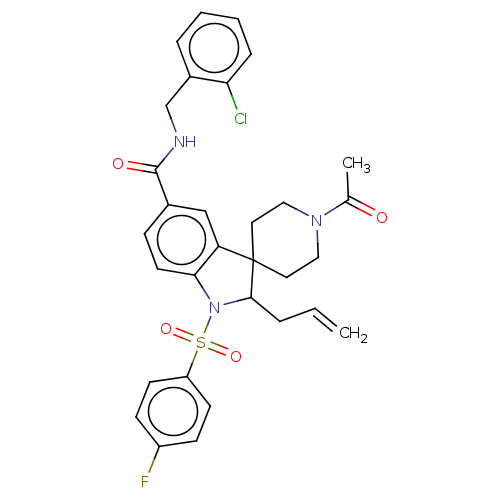 (CHEMBL4741994) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506187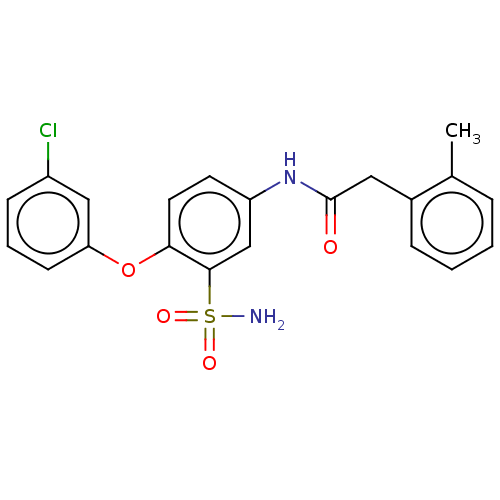 (CHEMBL4522504) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512726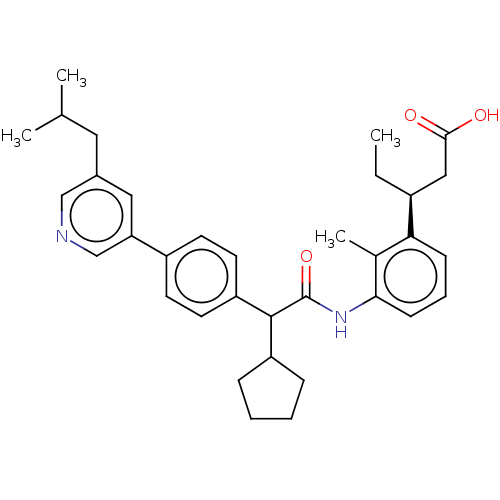 (CHEMBL4459950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506180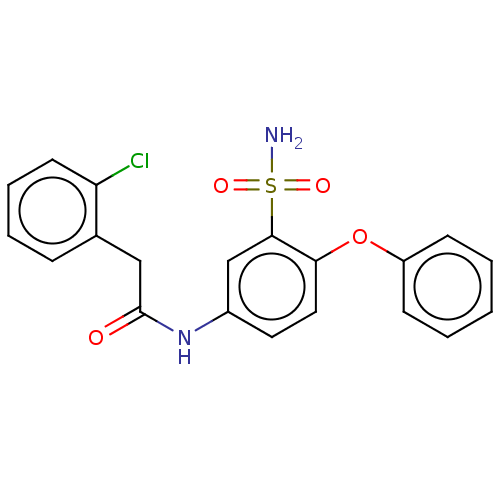 (CHEMBL4556573) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512715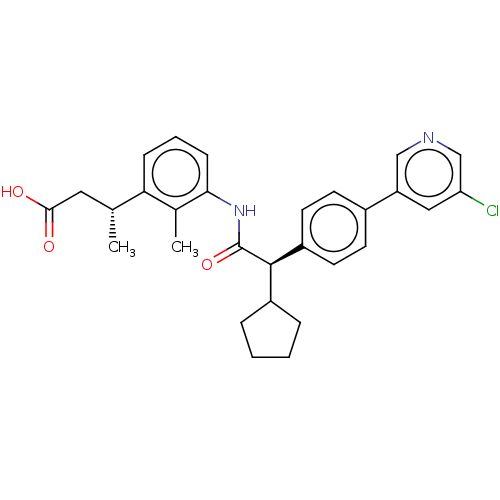 (CHEMBL4443717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548470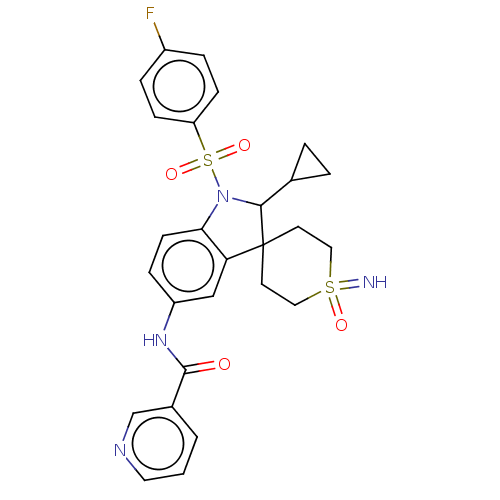 (CHEMBL4749974) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548470 (CHEMBL4749974) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50537004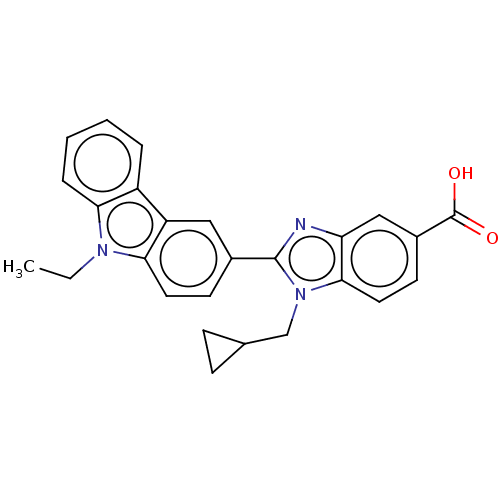 (CHEMBL4584103) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506201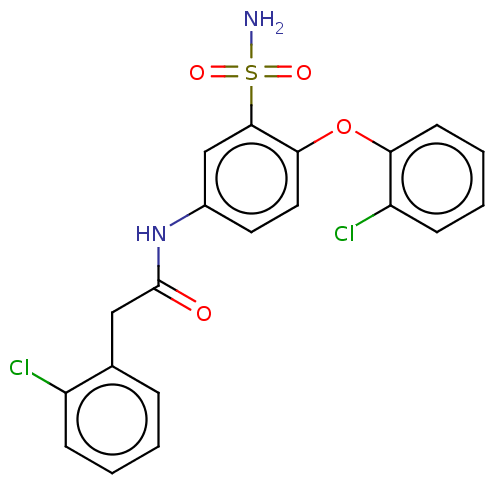 (CHEMBL4516176) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506178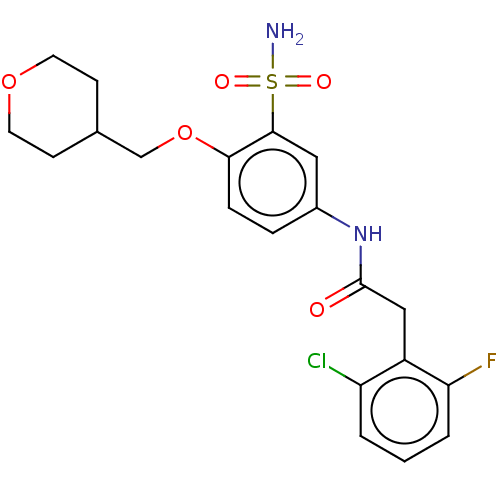 (CHEMBL4555657) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512700 (CHEMBL4443787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261892 (2-(9-Ethyl-6-methyl-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548472 (CHEMBL4749790) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548472 (CHEMBL4749790) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261766 (N-[(3-Chlorophenyl)sulphonyl]-2-(9-ethyl-9H-carbaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM314816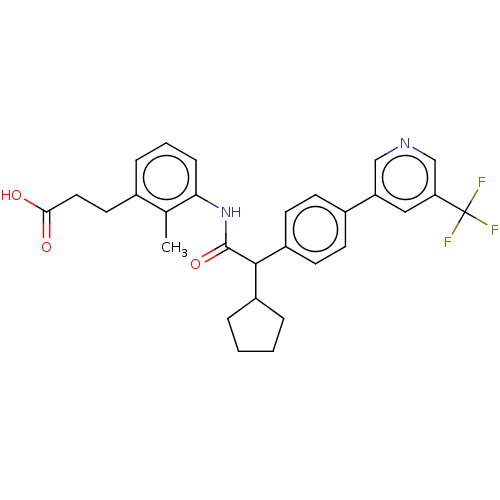 ((R/S) 3-{3-[(Cyclopentyl{4-[5-(trifluoromethyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506183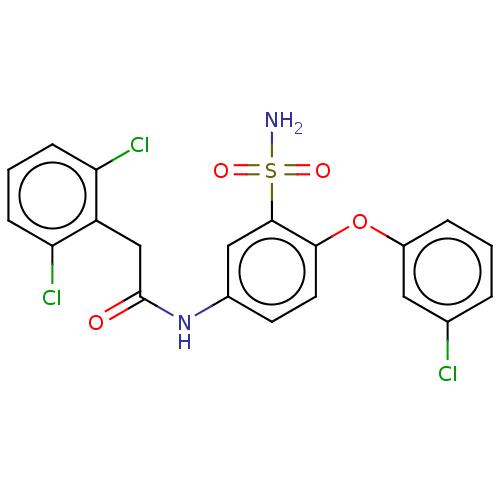 (CHEMBL4521017) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512718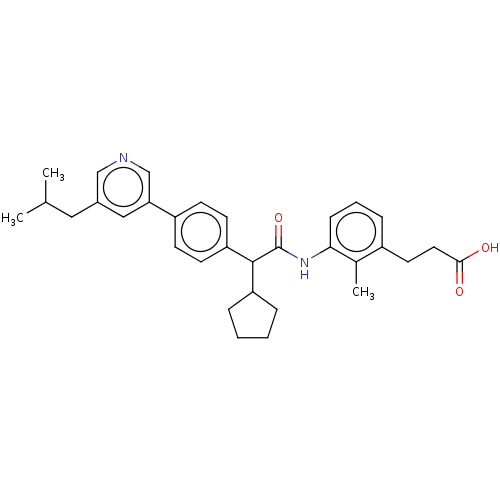 (CHEMBL4524932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50041550 (CHEMBL3358413) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50537016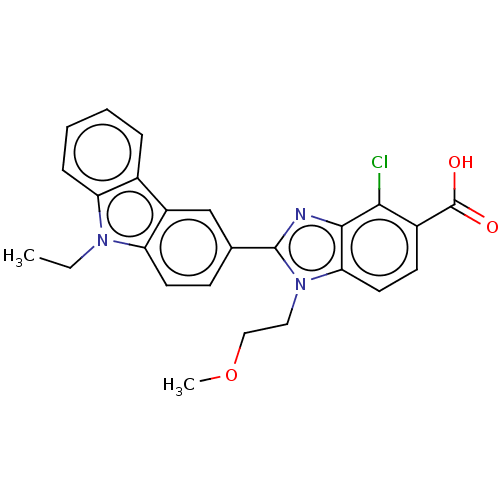 (CHEMBL4563081) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50537008 (CHEMBL4531968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548469 (CHEMBL4762368) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50537003 (CHEMBL4548032) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512714 (CHEMBL4454773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548475 (CHEMBL4747248) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506172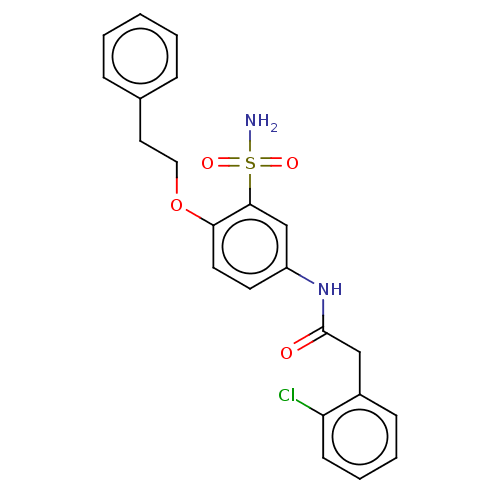 (CHEMBL4563994) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548463 (CHEMBL4781434) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM314817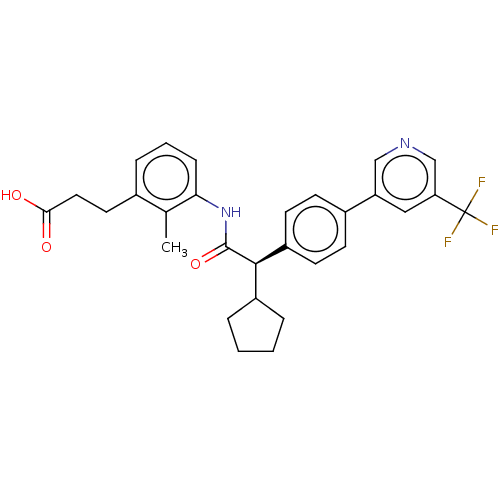 ((−) (R) 3-{3-[(Cyclopentyl{4-[5-(trifluorome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548468 (CHEMBL4747322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548488 (CHEMBL4780737) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512717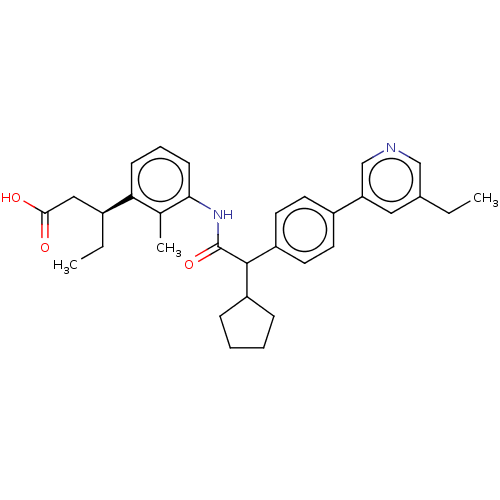 (CHEMBL4435689) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548493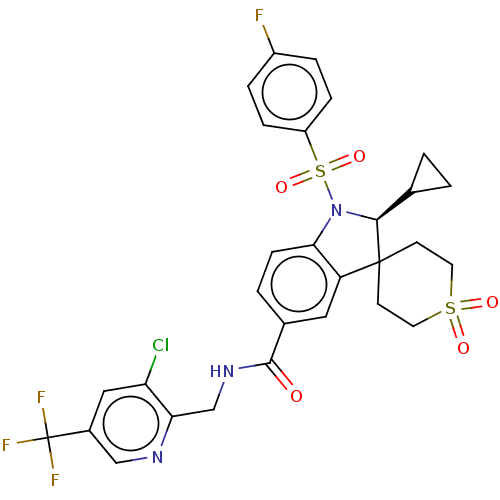 (BAY-784) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548488 (CHEMBL4780737) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50548486 (CHEMBL4741994) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01076 BindingDB Entry DOI: 10.7270/Q26D5XK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261867 (1-(2-Cyclopropylethyl)-2-(9-ethyl-9H-carbazol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assay | J Med Chem 62: 2541-2563 (2019) Article DOI: 10.1021/acs.jmedchem.8b01862 BindingDB Entry DOI: 10.7270/Q25H7KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50512716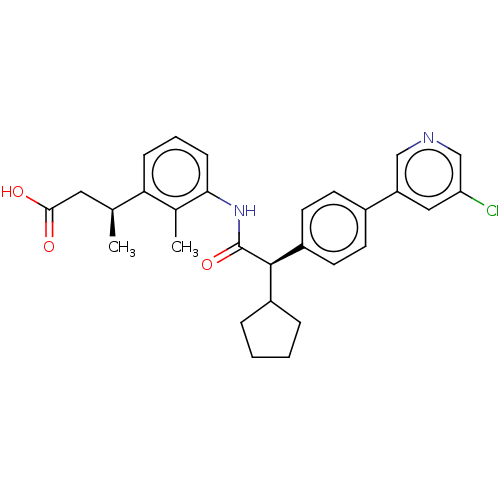 (CHEMBL4439662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of human recombinant FLAG-tagged PTGES expressed in baculovirus infected Hi-5 insect cells using prostaglandin H2 as substrate preincubate... | Bioorg Med Chem Lett 29: 2700-2705 (2019) Article DOI: 10.1016/j.bmcl.2019.07.007 BindingDB Entry DOI: 10.7270/Q2FX7DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 314 total ) | Next | Last >> |