

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254841 (US9487554, 28) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254826 (US9487554, 13) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414477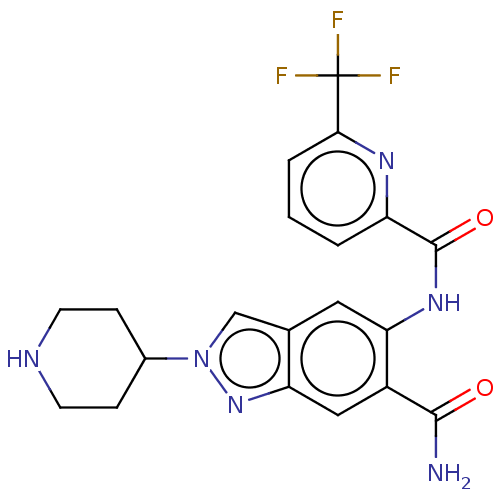 (2-(Piperidin-4-yl)-5-({[6-(trifluoromethyl)pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414479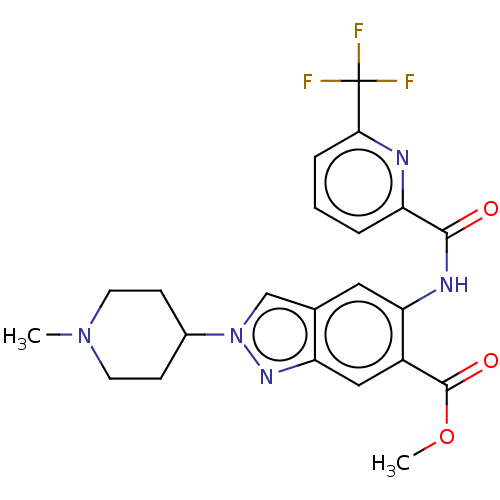 (Methyl 2-(1-methylpiperidin-4-yl)-5-({[6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414476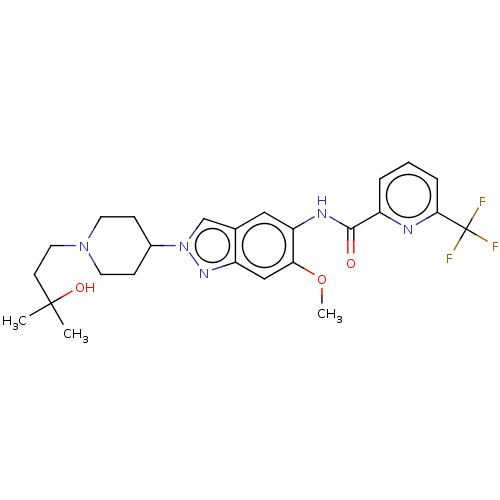 (N-{2-[1-(3-Hydroxy-3-methylbutyl)piperidin-4-yl]-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414444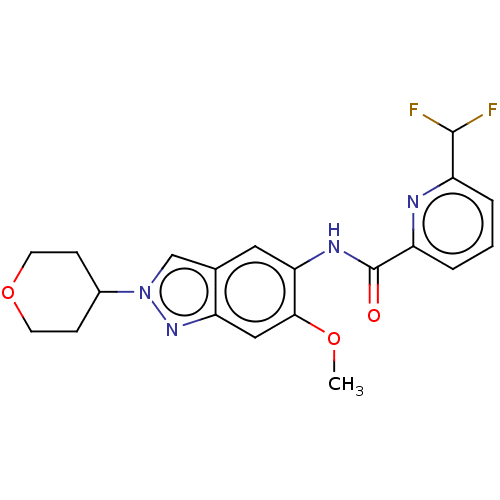 (6-(Difluoromethyl)-N-[6-methoxy-2-(tetrahydro-2H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414436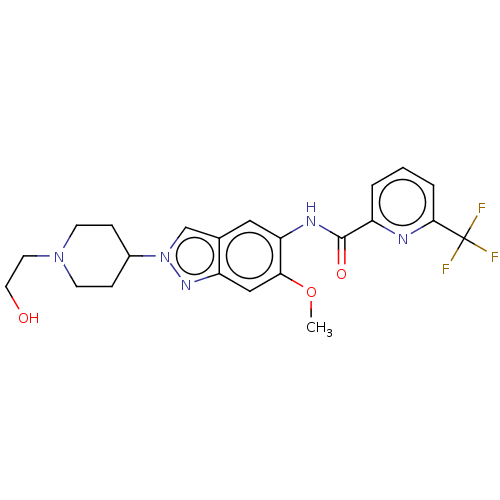 (N-{2-[1-(2-Hydroxyethyl)piperidin-4-yl]-6-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414432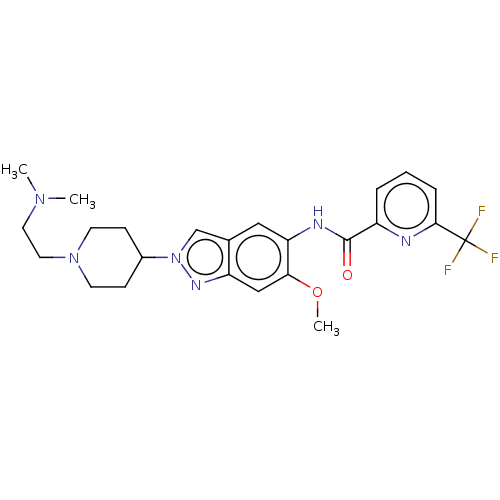 (N-(2-{1-[2-(Dimethylamino)ethyl]piperidin-4-yl}-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414423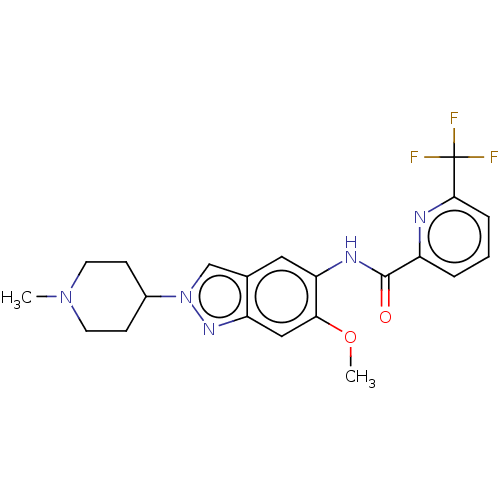 (N-[6-Methoxy-2-(1-methylpiperidin-4-yl)-2H-indazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414421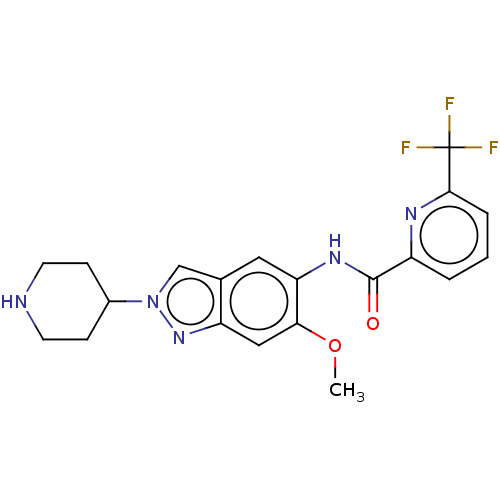 (N-[6-Methoxy-2-(piperidin-4-yl)-2H-indazol-5-yl]-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254825 (US9487554, 12) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254815 (US9487554, 2) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395298 (6-(Difluoromethyl)-N-[2-(3-hydroxy-3-methylbutyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | US Patent US10793545 (2020) BindingDB Entry DOI: 10.7270/Q29S1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395298 (6-(Difluoromethyl)-N-[2-(3-hydroxy-3-methylbutyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254814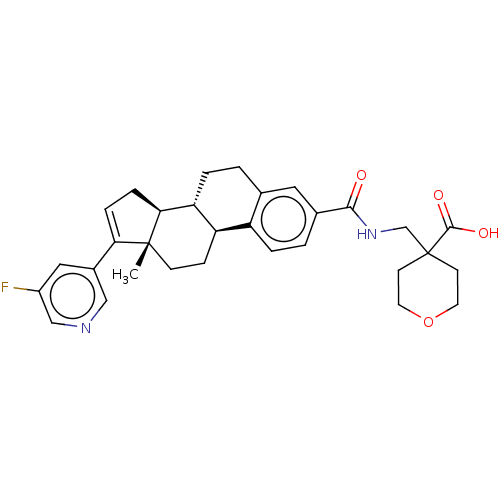 (US9487554, 1) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254815 (US9487554, 2) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM258453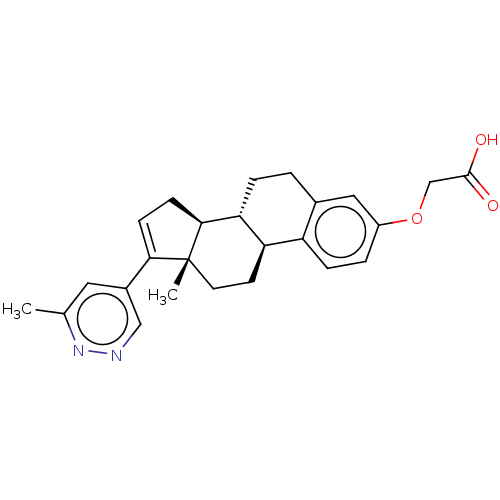 (US9512169, 7) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay, 50 nl of a 100-fold concentrated solution of the test substance in DMSO were pipetted into a black low-volume 384-well microtiter plat... | US Patent US9512169 (2016) BindingDB Entry DOI: 10.7270/Q24B3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453713 (2-(9-Ethyl-8-fluoro-6-methyl-9H-carbazol-3-yl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254825 (US9487554, 12) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254824 (US9487554, 11) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254826 (US9487554, 13) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453711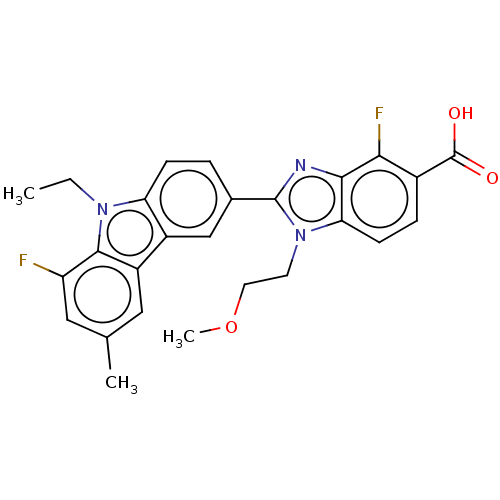 (2-(9-Ethyl-8-fluoro-6-methyl-9H-carbazol-3-yl)-4-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254836 (US9487554, 23) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254814 (US9487554, 1) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414481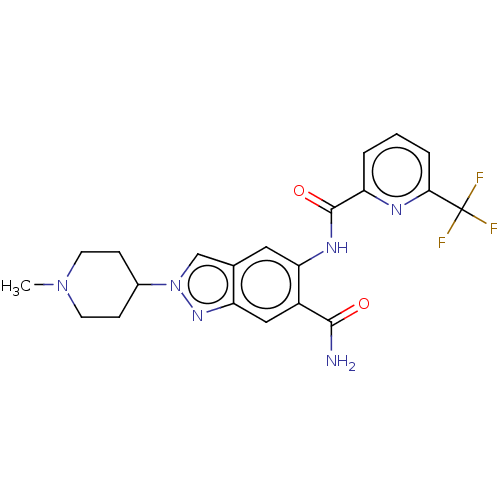 (2-(1-Methylpiperidin-4-yl)-5-({[6-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414482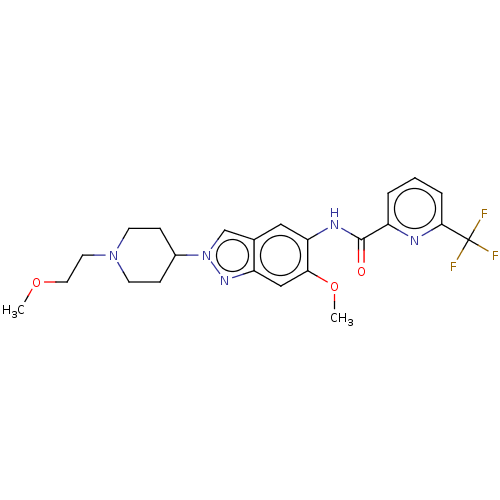 (N-{6-Methoxy-2-[1-(2-methoxyethyl)piperidin-4-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM390177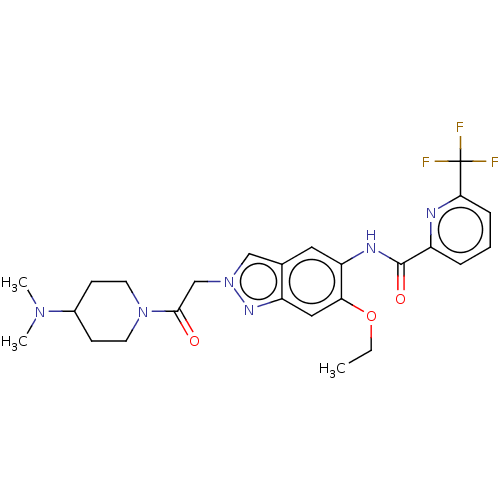 (US9951086, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | Bioorg Med Chem Lett 17: 3972-7 (2007) BindingDB Entry DOI: 10.7270/Q2GQ713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM390155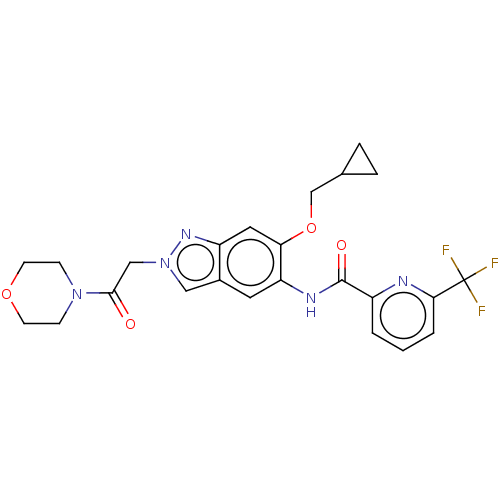 (US9951086, Example 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | Bioorg Med Chem Lett 17: 3972-7 (2007) BindingDB Entry DOI: 10.7270/Q2GQ713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414445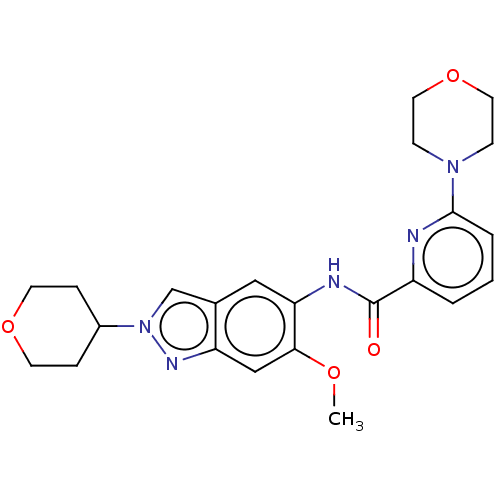 (N-[6-Methoxy-2-(tetrahydro-2H-pyran-4-yl)-2H-indaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414439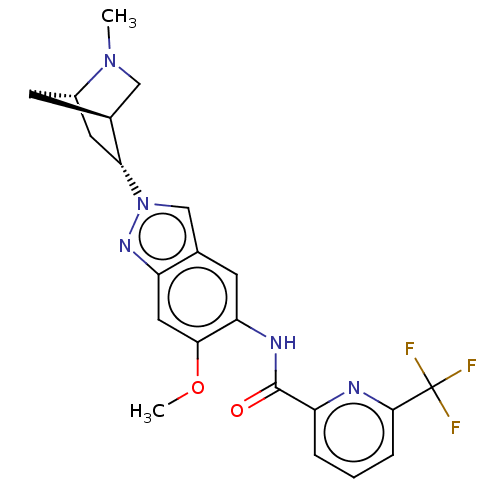 (US10435396, Example 18 | rel-N-{6-Methoxy-2-[(1S,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414435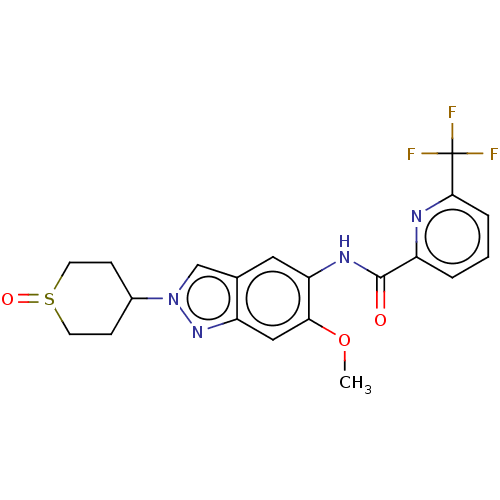 (US10435396, Example 12 | rac-N-[6-Methoxy-2-(1-oxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414428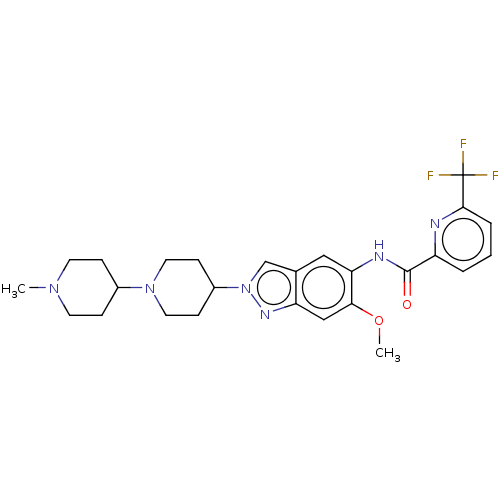 (N-[6-Methoxy-2-(1′-methyl-1,4′-bipiper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414424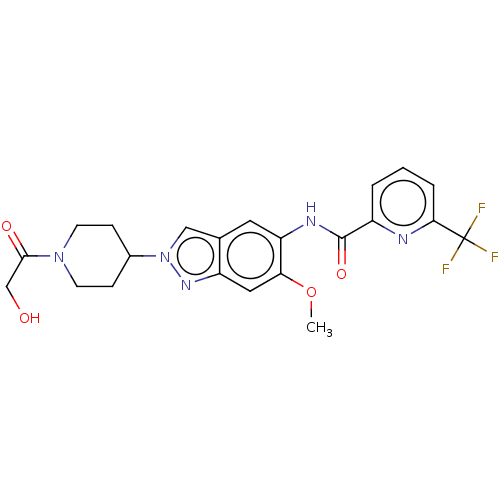 (N-[2-(1-Glycoloylpiperidin-4-yl)-6-methoxy-2H-inda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453704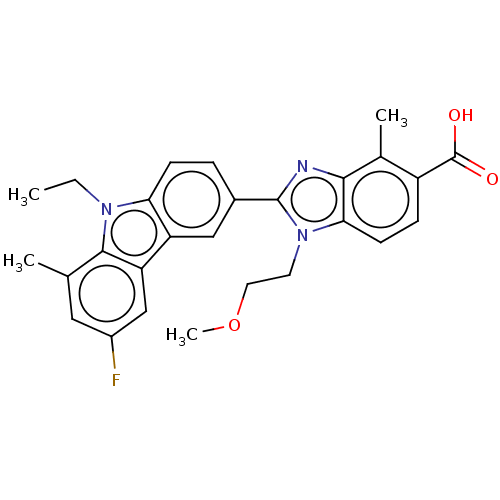 (2-(9-Ethyl-6-fluoro-8-methyl-9H-carbazol-3-yl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254824 (US9487554, 11) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453710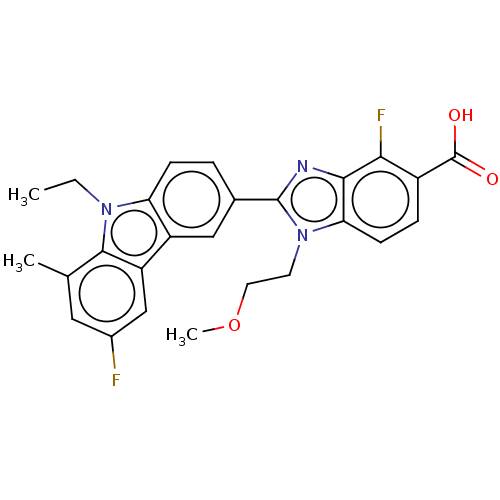 (2-(9-Ethyl-6-fluoro-8-methyl-9H-carbazol-3-yl)-4-f...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453702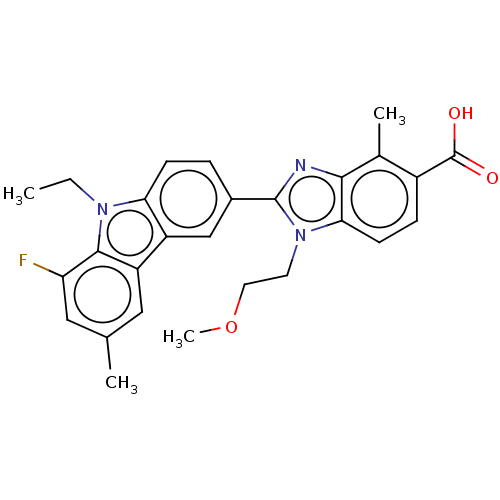 (2-(9-Ethyl-8-fluoro-6-methyl-9H-carbazol-3-yl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM258457 (US9512169, 11) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay, 50 nl of a 100-fold concentrated solution of the test substance in DMSO were pipetted into a black low-volume 384-well microtiter plat... | US Patent US9512169 (2016) BindingDB Entry DOI: 10.7270/Q24B3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM254842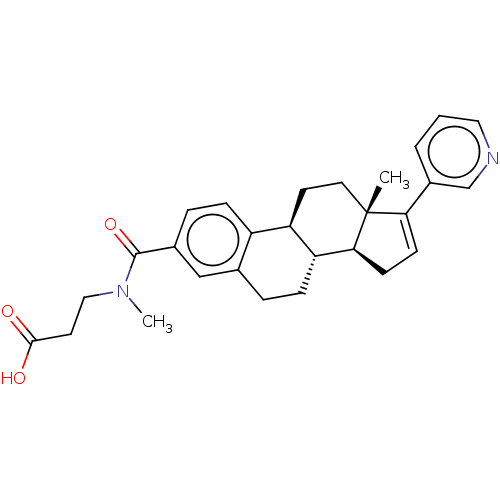 (US9487554, 29) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Intellectual Property GmbH; Bayer Pharma Aktiengesellschaft US Patent | Assay Description Essentially, the enzyme activity is measured by quantification of the Coumberol from Coumberone (Halim, M., Yee, D. J., and Sames, D., J. AM. CHEM. S... | US Patent US9487554 (2016) BindingDB Entry DOI: 10.7270/Q2KK99Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM258456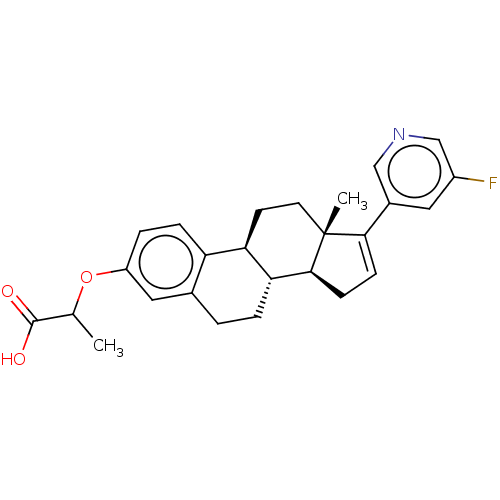 (US9512169, 10) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay, 50 nl of a 100-fold concentrated solution of the test substance in DMSO were pipetted into a black low-volume 384-well microtiter plat... | US Patent US9512169 (2016) BindingDB Entry DOI: 10.7270/Q24B3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM453715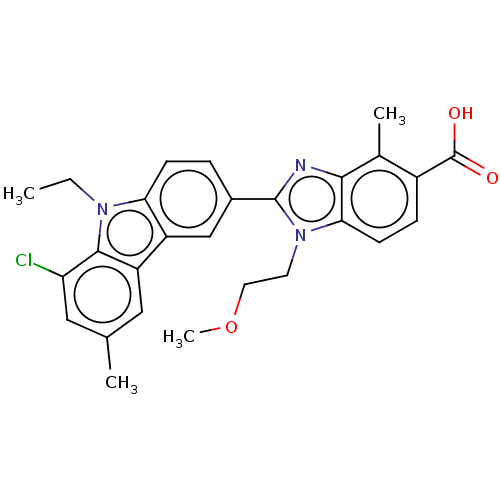 (2-(8-Chloro-9-ethyl-6-methyl-9H-carbazol-3-yl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th... | US Patent US10730856 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM258459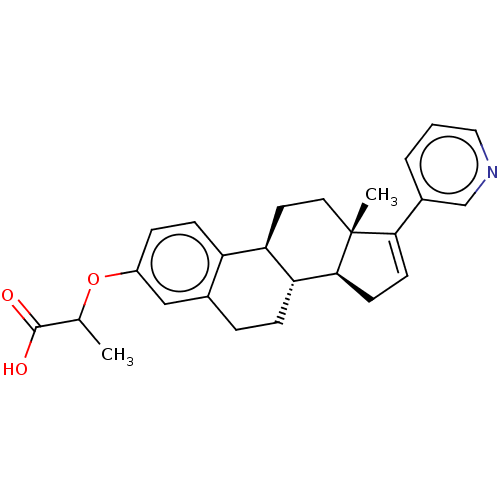 (US9512169, 13) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay, 50 nl of a 100-fold concentrated solution of the test substance in DMSO were pipetted into a black low-volume 384-well microtiter plat... | US Patent US9512169 (2016) BindingDB Entry DOI: 10.7270/Q24B3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414419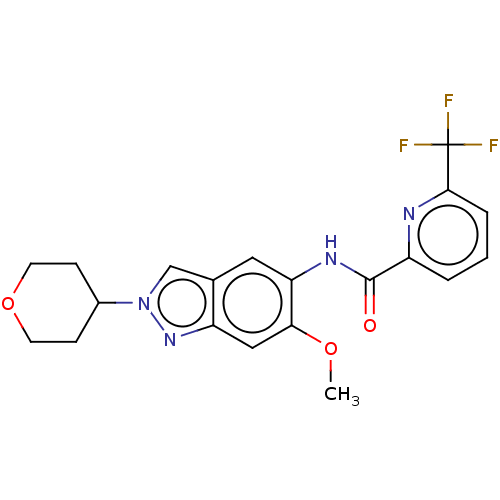 (N-[6-Methoxy-2-(tetrahydro-2H-pyran-4-yl)-2H-indaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM390154 (US9951086, Example 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | Bioorg Med Chem Lett 17: 3972-7 (2007) BindingDB Entry DOI: 10.7270/Q2GQ713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414469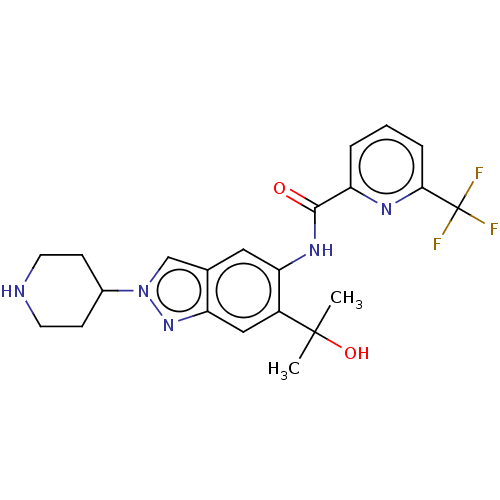 (N-[6-(2-Hydroxypropan-2-yl)-2-(piperidin-4-yl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414457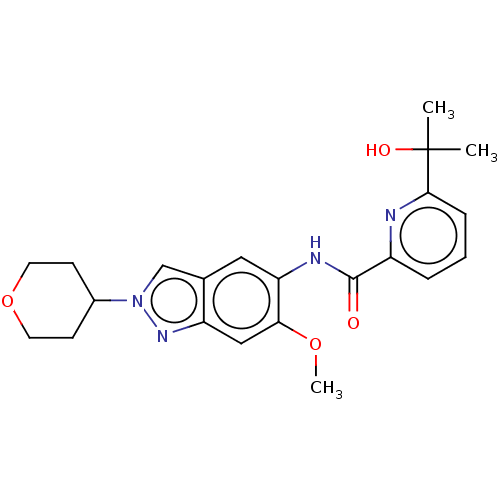 (6-(2-Hydroxypropan-2-yl)-N-[6-methoxy-2-(tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414442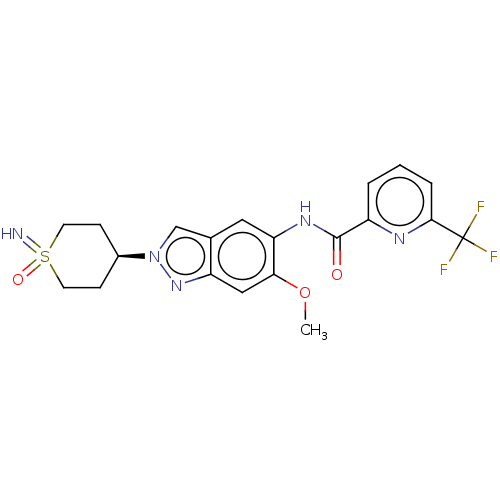 (N-[2-(1-Imino-1-oxidohexahydro-1λ4-thiopyran-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414434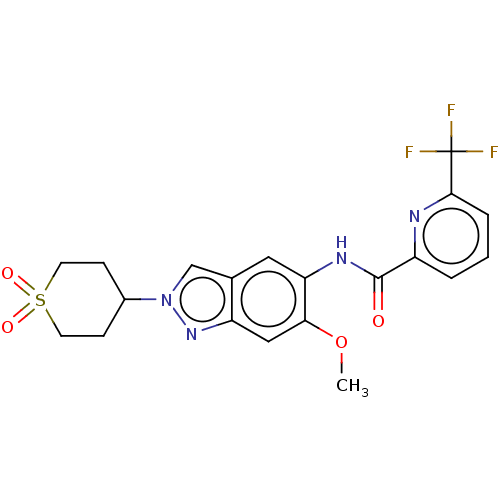 (N-[2-(1,1-Dioxidotetrahydro-2H-thiopyran-4-yl)-6-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM414433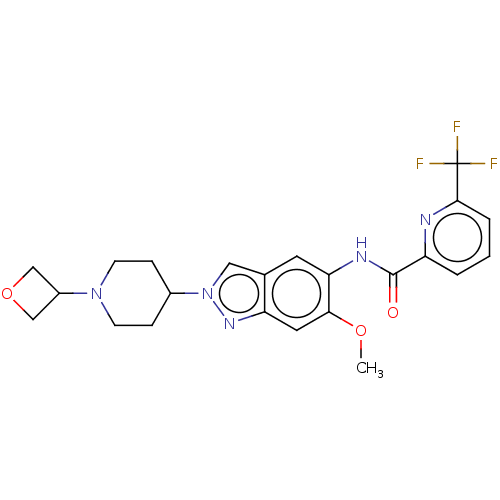 (N-{6-Methoxy-2-[1-(oxetan-3-yl)piperidin-4-yl]-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiegesellschaft US Patent | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM solution of the test substance in DMSO.... | US Patent US10435396 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM395296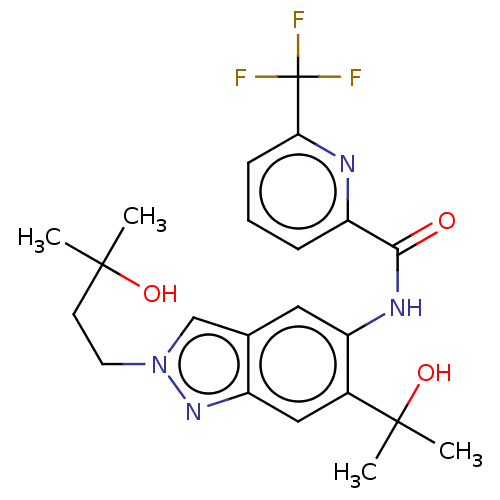 (N-[2-(3-Hydroxy-3-methylbutyl)-6-(2-hydroxypropan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description For the assay, 11 different concentrations in the range from 20 μM to 0.073 nM were prepared from a 2 mM DMSO solution of the test substance. 50... | Bioorg Med Chem Lett 19: 3550-4 (2009) BindingDB Entry DOI: 10.7270/Q2J67K7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 612 total ) | Next | Last >> |