 Found 86 hits with Last Name = 'balasubramanian' and Initial = 'v'
Found 86 hits with Last Name = 'balasubramanian' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525789
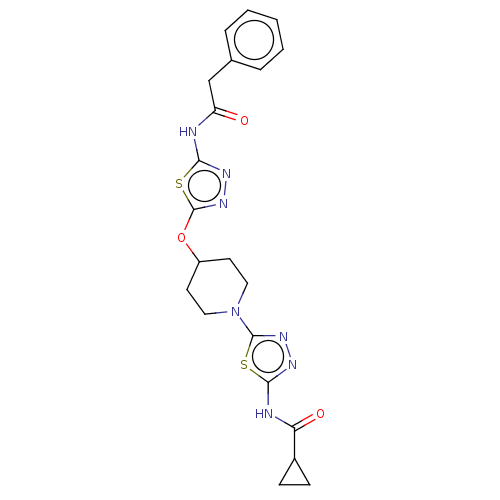
(CHEMBL4469854)Show SMILES O=C(Cc1ccccc1)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)C3CC3)s2)s1 Show InChI InChI=1S/C21H23N7O3S2/c29-16(12-13-4-2-1-3-5-13)22-18-25-27-21(33-18)31-15-8-10-28(11-9-15)20-26-24-19(32-20)23-17(30)14-6-7-14/h1-5,14-15H,6-12H2,(H,22,25,29)(H,23,24,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525789

(CHEMBL4469854)Show SMILES O=C(Cc1ccccc1)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)C3CC3)s2)s1 Show InChI InChI=1S/C21H23N7O3S2/c29-16(12-13-4-2-1-3-5-13)22-18-25-27-21(33-18)31-15-8-10-28(11-9-15)20-26-24-19(32-20)23-17(30)14-6-7-14/h1-5,14-15H,6-12H2,(H,22,25,29)(H,23,24,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525793
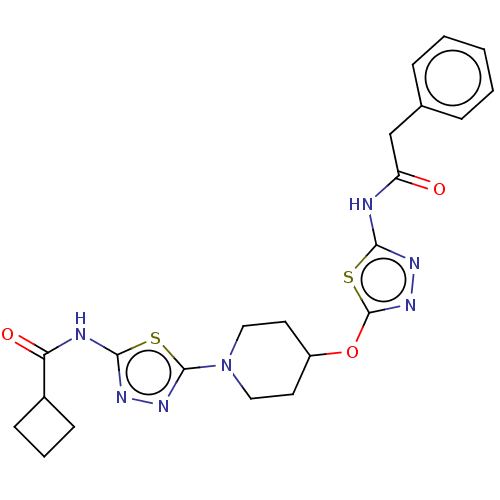
(CHEMBL4436129)Show SMILES O=C(Cc1ccccc1)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)C3CCC3)s2)s1 Show InChI InChI=1S/C22H25N7O3S2/c30-17(13-14-5-2-1-3-6-14)23-19-26-28-22(34-19)32-16-9-11-29(12-10-16)21-27-25-20(33-21)24-18(31)15-7-4-8-15/h1-3,5-6,15-16H,4,7-13H2,(H,23,26,30)(H,24,25,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525793

(CHEMBL4436129)Show SMILES O=C(Cc1ccccc1)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)C3CCC3)s2)s1 Show InChI InChI=1S/C22H25N7O3S2/c30-17(13-14-5-2-1-3-6-14)23-19-26-28-22(34-19)32-16-9-11-29(12-10-16)21-27-25-20(33-21)24-18(31)15-7-4-8-15/h1-3,5-6,15-16H,4,7-13H2,(H,23,26,30)(H,24,25,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525797
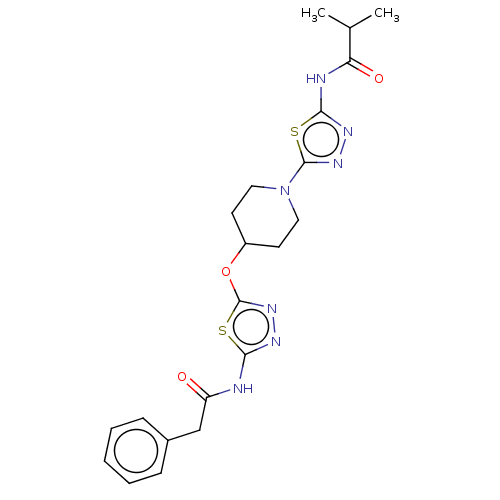
(CHEMBL4524344)Show SMILES CC(C)C(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C21H25N7O3S2/c1-13(2)17(30)23-19-24-26-20(32-19)28-10-8-15(9-11-28)31-21-27-25-18(33-21)22-16(29)12-14-6-4-3-5-7-14/h3-7,13,15H,8-12H2,1-2H3,(H,22,25,29)(H,23,24,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525797

(CHEMBL4524344)Show SMILES CC(C)C(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C21H25N7O3S2/c1-13(2)17(30)23-19-24-26-20(32-19)28-10-8-15(9-11-28)31-21-27-25-18(33-21)22-16(29)12-14-6-4-3-5-7-14/h3-7,13,15H,8-12H2,1-2H3,(H,22,25,29)(H,23,24,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525795
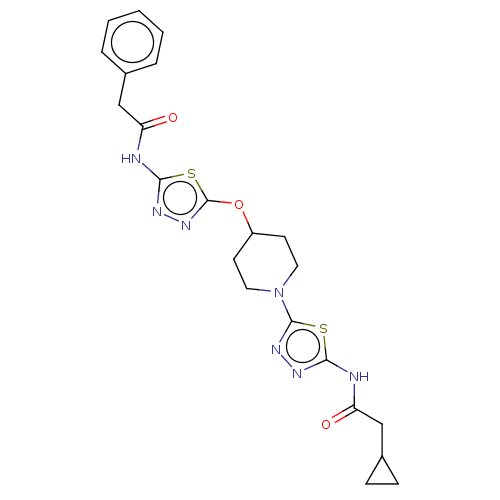
(CHEMBL4459383)Show SMILES O=C(CC1CC1)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C22H25N7O3S2/c30-17(12-14-4-2-1-3-5-14)24-20-26-28-22(34-20)32-16-8-10-29(11-9-16)21-27-25-19(33-21)23-18(31)13-15-6-7-15/h1-5,15-16H,6-13H2,(H,23,25,31)(H,24,26,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525795

(CHEMBL4459383)Show SMILES O=C(CC1CC1)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C22H25N7O3S2/c30-17(12-14-4-2-1-3-5-14)24-20-26-28-22(34-20)32-16-8-10-29(11-9-16)21-27-25-19(33-21)23-18(31)13-15-6-7-15/h1-5,15-16H,6-13H2,(H,23,25,31)(H,24,26,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525790
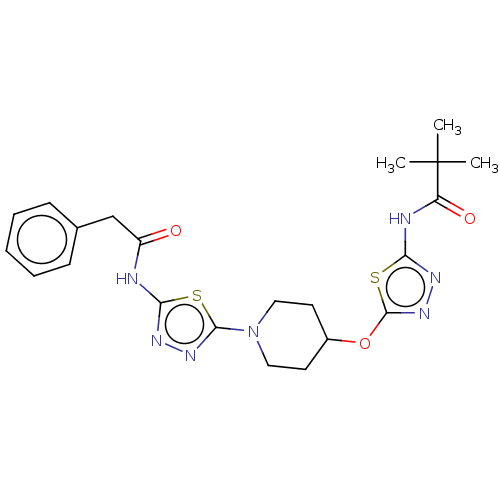
(CHEMBL4578823)Show SMILES CC(C)(C)C(=O)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C22H27N7O3S2/c1-22(2,3)17(31)24-19-26-28-21(34-19)32-15-9-11-29(12-10-15)20-27-25-18(33-20)23-16(30)13-14-7-5-4-6-8-14/h4-8,15H,9-13H2,1-3H3,(H,23,25,30)(H,24,26,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525790

(CHEMBL4578823)Show SMILES CC(C)(C)C(=O)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C22H27N7O3S2/c1-22(2,3)17(31)24-19-26-28-21(34-19)32-15-9-11-29(12-10-15)20-27-25-18(33-20)23-16(30)13-14-7-5-4-6-8-14/h4-8,15H,9-13H2,1-3H3,(H,23,25,30)(H,24,26,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525796
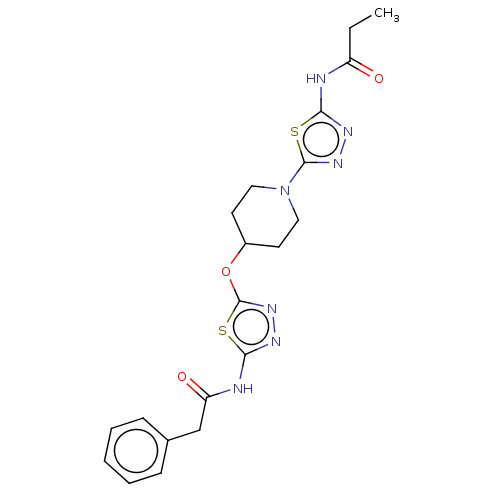
(CHEMBL4454551)Show SMILES CCC(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C20H23N7O3S2/c1-2-15(28)21-17-23-25-19(31-17)27-10-8-14(9-11-27)30-20-26-24-18(32-20)22-16(29)12-13-6-4-3-5-7-13/h3-7,14H,2,8-12H2,1H3,(H,21,23,28)(H,22,24,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525796

(CHEMBL4454551)Show SMILES CCC(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C20H23N7O3S2/c1-2-15(28)21-17-23-25-19(31-17)27-10-8-14(9-11-27)30-20-26-24-18(32-20)22-16(29)12-13-6-4-3-5-7-13/h3-7,14H,2,8-12H2,1H3,(H,21,23,28)(H,22,24,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50149992
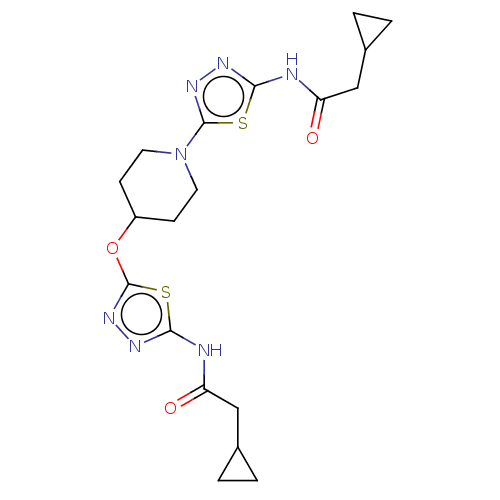
(CHEMBL3769897)Show SMILES O=C(CC1CC1)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)CC3CC3)s2)s1 Show InChI InChI=1S/C23H22BrN5O5S/c1-14(30)28-19(23(32)29-21-11-10-17(24)12-26-21)13-27-22(31)16-8-6-15(7-9-16)18-4-2-3-5-20(18)35(25,33)34/h2-12,19H,13H2,1H3,(H,27,31)(H,28,30)(H2,25,33,34)(H,26,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50149992

(CHEMBL3769897)Show SMILES O=C(CC1CC1)Nc1nnc(OC2CCN(CC2)c2nnc(NC(=O)CC3CC3)s2)s1 Show InChI InChI=1S/C23H22BrN5O5S/c1-14(30)28-19(23(32)29-21-11-10-17(24)12-26-21)13-27-22(31)16-8-6-15(7-9-16)18-4-2-3-5-20(18)35(25,33)34/h2-12,19H,13H2,1H3,(H,27,31)(H,28,30)(H2,25,33,34)(H,26,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525792
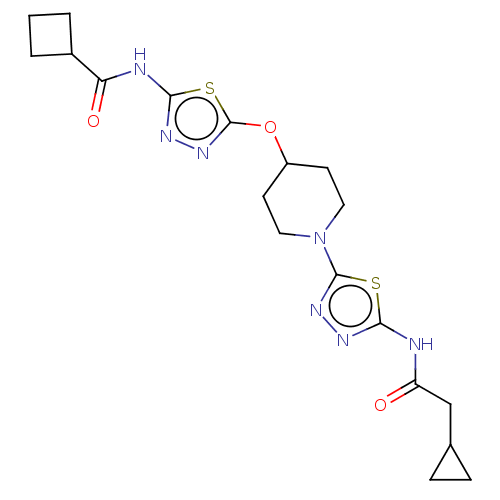
(CHEMBL4588969)Show SMILES O=C(CC1CC1)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)C2CCC2)s1 Show InChI InChI=1S/C19H25N7O3S2/c27-14(10-11-4-5-11)20-16-22-24-18(30-16)26-8-6-13(7-9-26)29-19-25-23-17(31-19)21-15(28)12-2-1-3-12/h11-13H,1-10H2,(H,20,22,27)(H,21,23,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525792

(CHEMBL4588969)Show SMILES O=C(CC1CC1)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)C2CCC2)s1 Show InChI InChI=1S/C19H25N7O3S2/c27-14(10-11-4-5-11)20-16-22-24-18(30-16)26-8-6-13(7-9-26)29-19-25-23-17(31-19)21-15(28)12-2-1-3-12/h11-13H,1-10H2,(H,20,22,27)(H,21,23,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525794
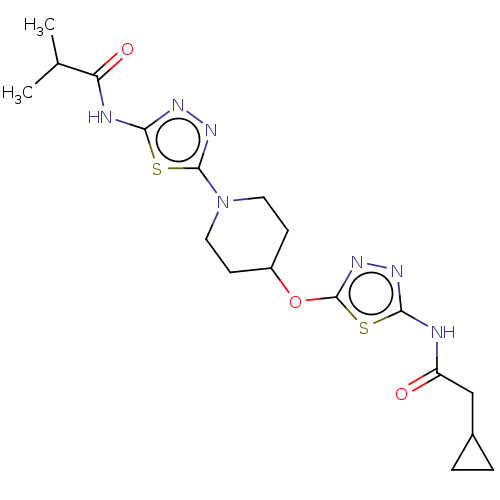
(CHEMBL4454980)Show SMILES CC(C)C(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)CC2CC2)s1 Show InChI InChI=1S/C18H25N7O3S2/c1-10(2)14(27)20-16-21-23-17(29-16)25-7-5-12(6-8-25)28-18-24-22-15(30-18)19-13(26)9-11-3-4-11/h10-12H,3-9H2,1-2H3,(H,19,22,26)(H,20,21,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525794

(CHEMBL4454980)Show SMILES CC(C)C(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)CC2CC2)s1 Show InChI InChI=1S/C18H25N7O3S2/c1-10(2)14(27)20-16-21-23-17(29-16)25-7-5-12(6-8-25)28-18-24-22-15(30-18)19-13(26)9-11-3-4-11/h10-12H,3-9H2,1-2H3,(H,19,22,26)(H,20,21,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525791
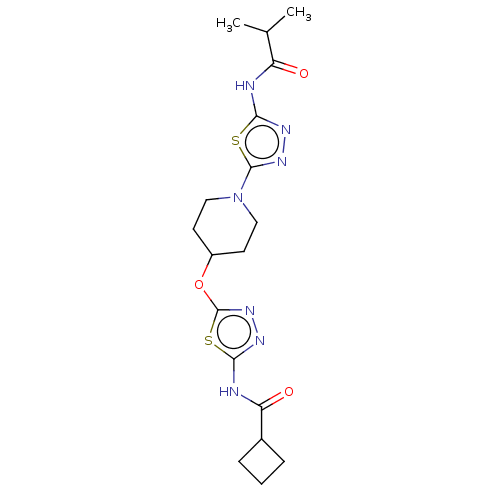
(CHEMBL4441341)Show SMILES CC(C)C(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)C2CCC2)s1 Show InChI InChI=1S/C18H25N7O3S2/c1-10(2)13(26)19-15-21-23-17(29-15)25-8-6-12(7-9-25)28-18-24-22-16(30-18)20-14(27)11-4-3-5-11/h10-12H,3-9H2,1-2H3,(H,19,21,26)(H,20,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50525791

(CHEMBL4441341)Show SMILES CC(C)C(=O)Nc1nnc(s1)N1CCC(CC1)Oc1nnc(NC(=O)C2CCC2)s1 Show InChI InChI=1S/C18H25N7O3S2/c1-10(2)13(26)19-15-21-23-17(29-15)25-8-6-12(7-9-25)28-18-24-22-16(30-18)20-14(27)11-4-3-5-11/h10-12H,3-9H2,1-2H3,(H,19,21,26)(H,20,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged GAC (72 to 603 residues) expressed in Escherichia coli using glutamine as substrate incubated for 10 mins |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126632
BindingDB Entry DOI: 10.7270/Q29W0JX3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020413
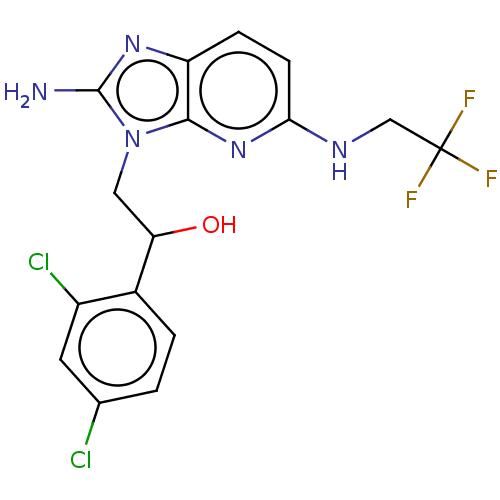
(CHEMBL3289807)Show SMILES Nc1nc2ccc(NCC(F)(F)F)nc2n1CC(O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H14Cl2F3N5O/c17-8-1-2-9(10(18)5-8)12(27)6-26-14-11(24-15(26)22)3-4-13(25-14)23-7-16(19,20)21/h1-5,12,27H,6-7H2,(H2,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020414

(CHEMBL3289797)Show InChI InChI=1S/C14H15Cl2N3O/c15-9-3-4-10(11(16)5-9)13(20)7-19-6-12(8-1-2-8)18-14(19)17/h3-6,8,13,20H,1-2,7H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020415
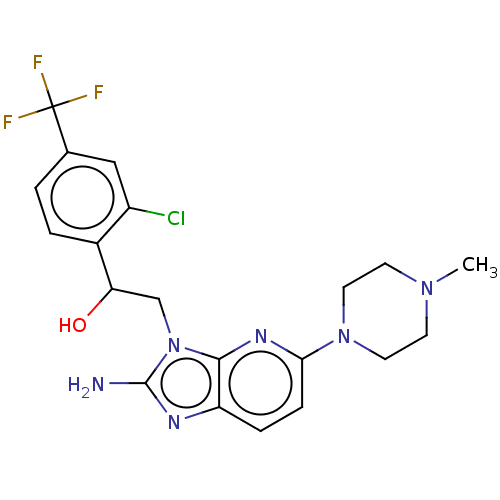
(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499994
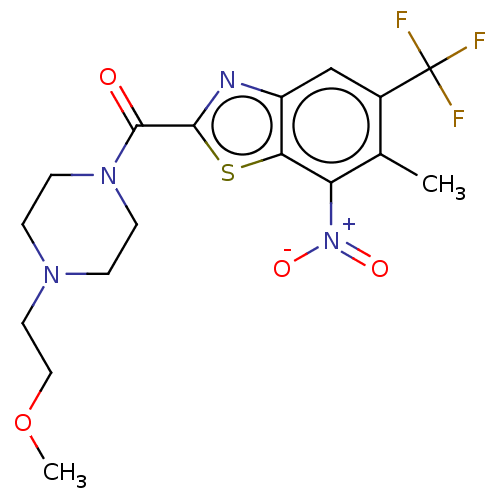
(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020416
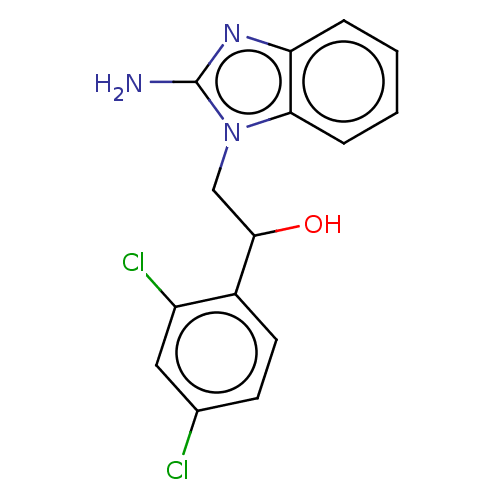
(CHEMBL3289799)Show InChI InChI=1S/C15H13Cl2N3O/c16-9-5-6-10(11(17)7-9)14(21)8-20-13-4-2-1-3-12(13)19-15(20)18/h1-7,14,21H,8H2,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020417

(CHEMBL3289794)Show InChI InChI=1S/C11H11Cl2N3O/c12-8-3-1-2-7(10(8)13)9(17)6-16-5-4-15-11(16)14/h1-5,9,17H,6H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499995
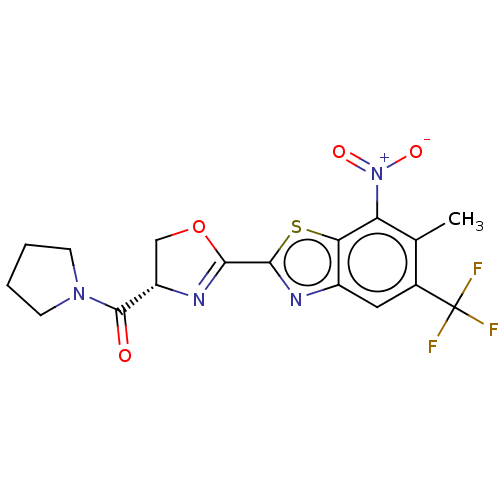
(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data