

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464596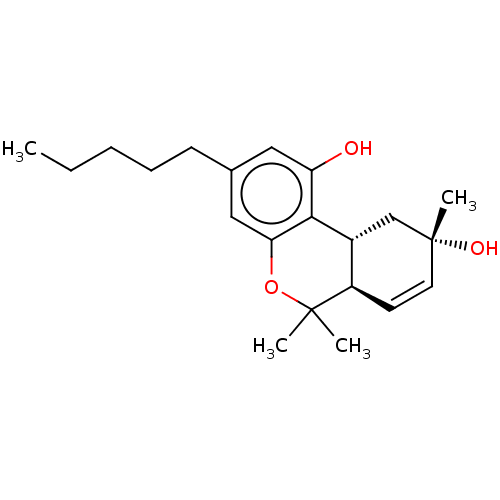 (CHEMBL4282822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464593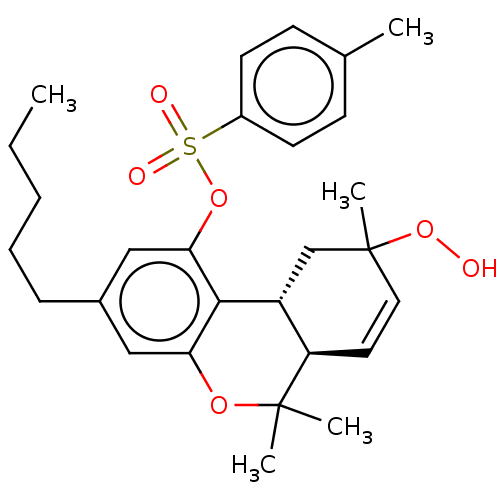 (CHEMBL4291438) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464593 (CHEMBL4291438) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464596 (CHEMBL4282822) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464593 (CHEMBL4291438) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464601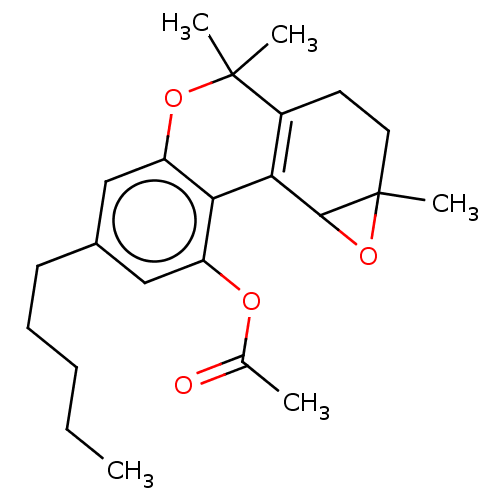 (CHEMBL4290707) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464602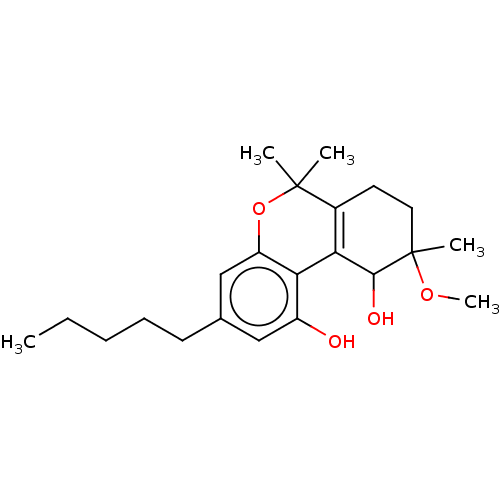 (CHEMBL4286213) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464597 (CHEMBL4285088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464601 (CHEMBL4290707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 464 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464596 (CHEMBL4282822) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464597 (CHEMBL4285088) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 512 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464602 (CHEMBL4286213) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 538 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464599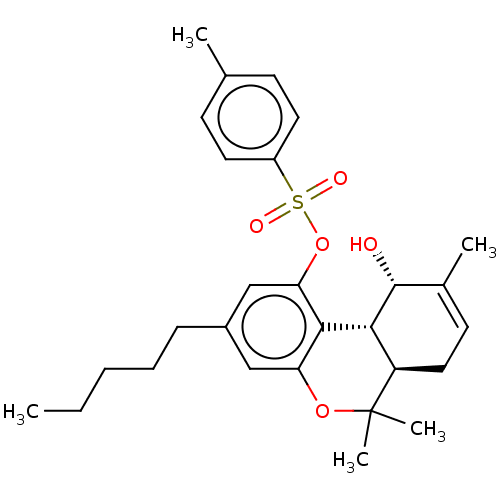 (CHEMBL4292923) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 552 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464599 (CHEMBL4292923) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 642 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464593 (CHEMBL4291438) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 876 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464594 (CHEMBL4289543) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 919 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464595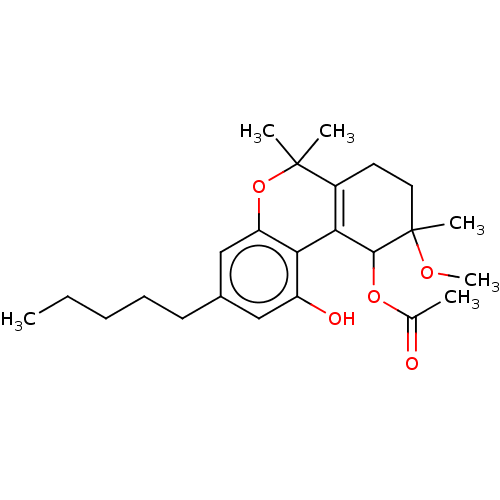 (CHEMBL4294111) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464600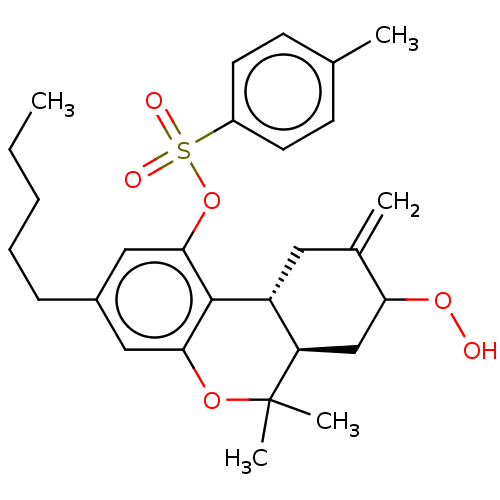 (CHEMBL4283589) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464595 (CHEMBL4294111) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464594 (CHEMBL4289543) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464600 (CHEMBL4283589) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464596 (CHEMBL4282822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464596 (CHEMBL4282822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464598 (CHEMBL4281658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50464593 (CHEMBL4291438) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387452 (N3-(3-Chloro-4- fluorophenyl)- 5-methoxy-7- methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387576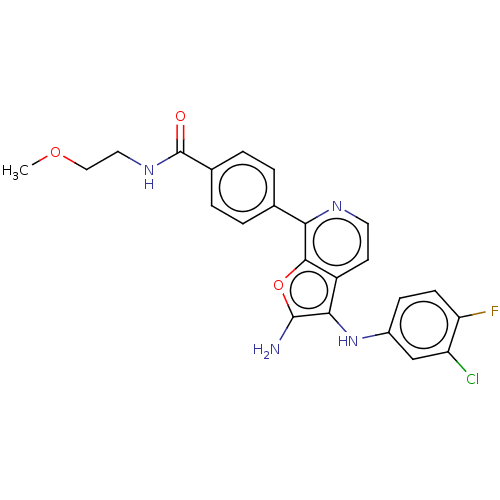 (4-(2-Amino-3- ((3-chloro-4- fluorophenyl) amino)fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387577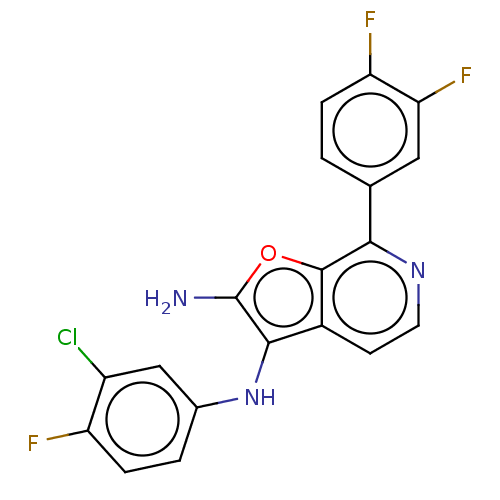 (N3-(3-chloro-4- fluorophenyl)- 7-(3,4- difluorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387578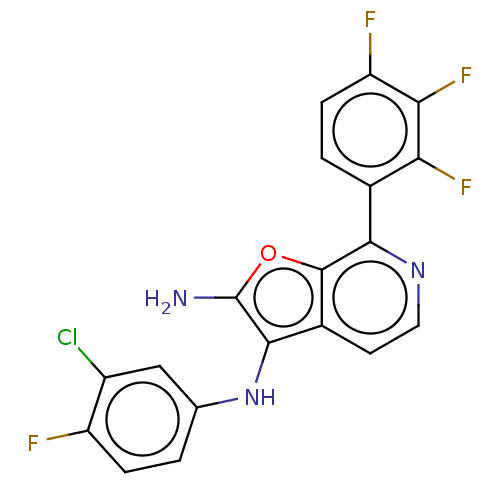 (N3-(3-Chloro-4- fluorophenyl)- 7-(2,3,4- trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387579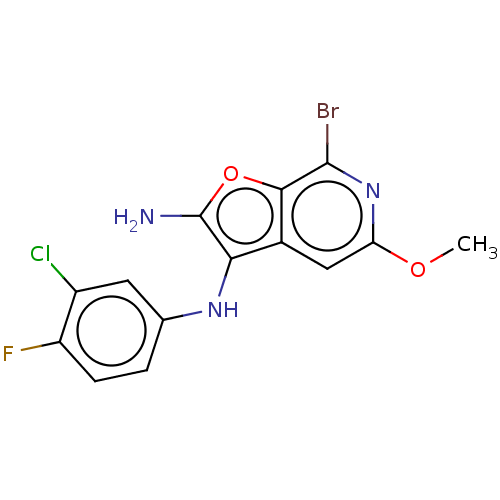 (7-Bromo-N3-(3- chloro-4- fluorophenyl)- 5-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387580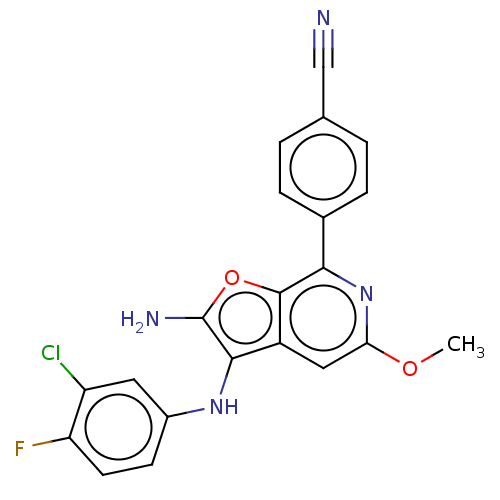 (4-(2-Amino-3- ((3-chloro-4- fluorophenyl) amino)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387582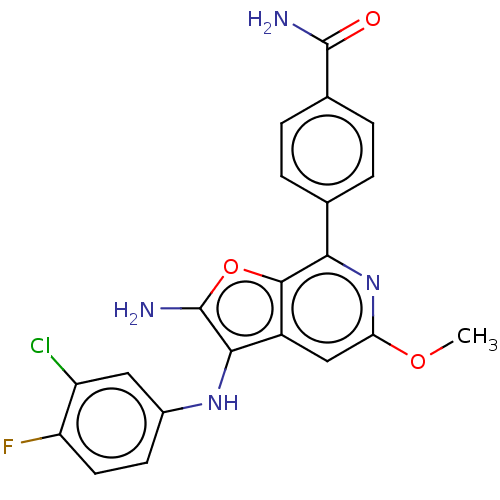 (4-(2-amino-3- ((3-chloro-4- fluorophenyl) amino)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387585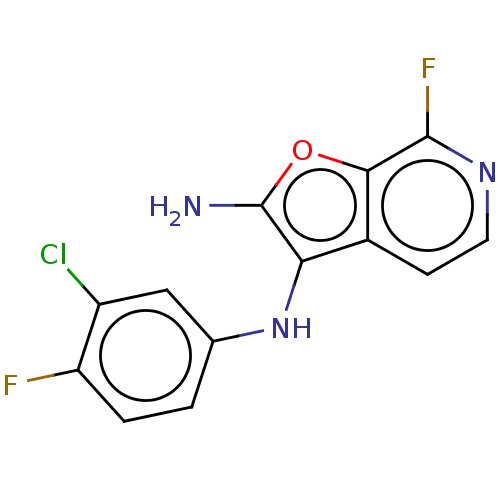 (N3-(3-chloro-4- fluorophenyl)- 7-fluorofuro [2,3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387586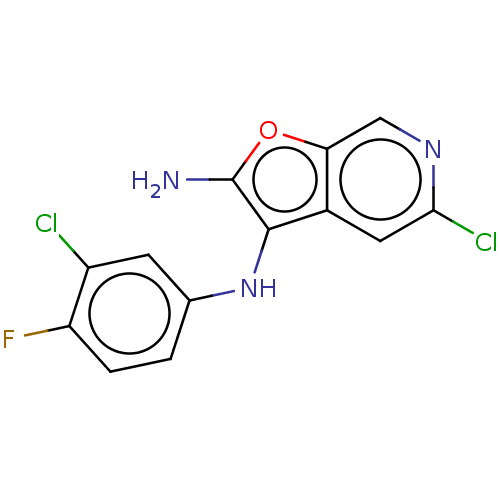 (5-Chloro-N3- (3-chloro-4- fluorophenyl) furo[2,3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387587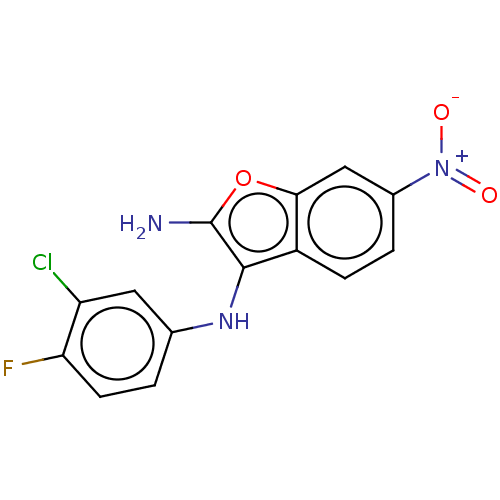 (N3-(3-chloro-4- fluorophenyl)- 6-nitrobenzo- furan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387588 (N3-(3-Chloro-4- fluorophenyl)- 5-fluoro-7- (pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387589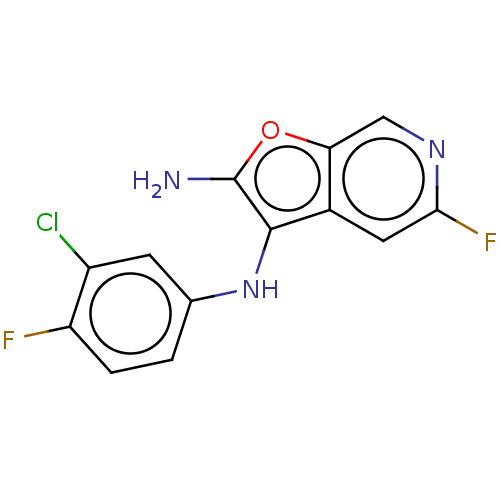 (N3-(3-Chloro-4- fluorophenyl)- 5-fluorofuro [2,3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387590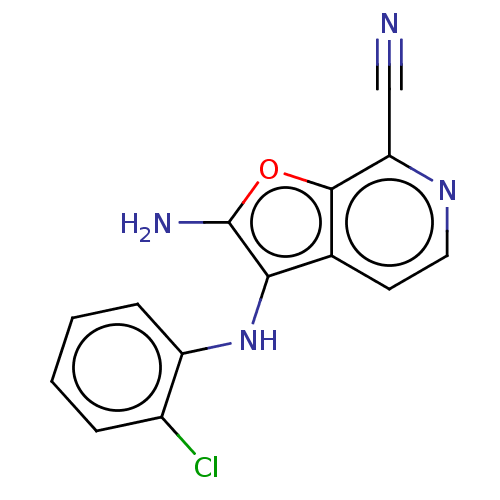 (2-Amino-3-((2- chlorophenyl) amino)furo [2,3-c]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387591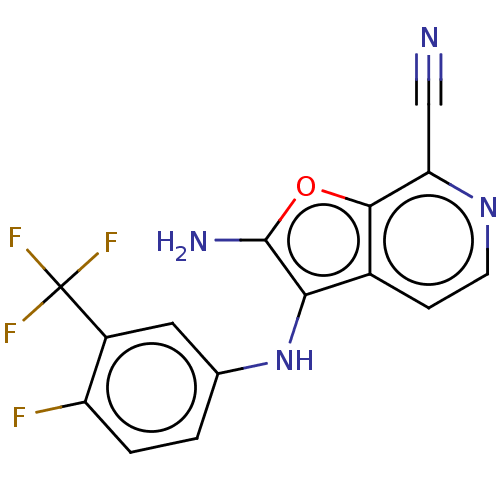 (2-Amino-3- ((4-fluoro-3- (trifluoro- methyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387592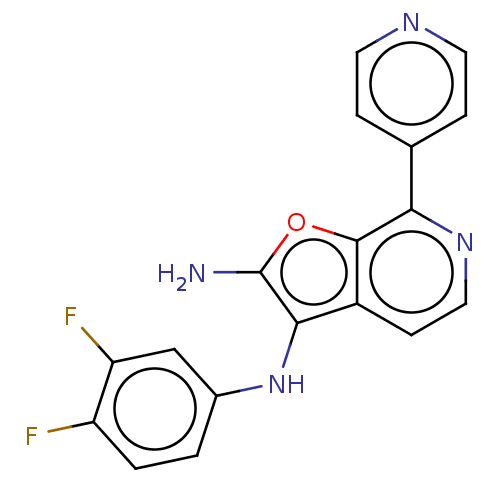 (N3-(3,4- difluoro- phenyl)-7- (pyridin-4- yl)furo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387603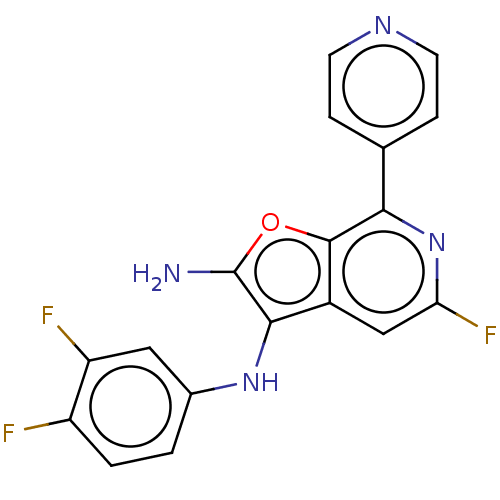 (N3-(3,4- Difluoro- phenyl)-5- fluoro-7- (pyridin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 333 total ) | Next | Last >> |