 Found 60 hits with Last Name = 'choung' and Initial = 'w'
Found 60 hits with Last Name = 'choung' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467129
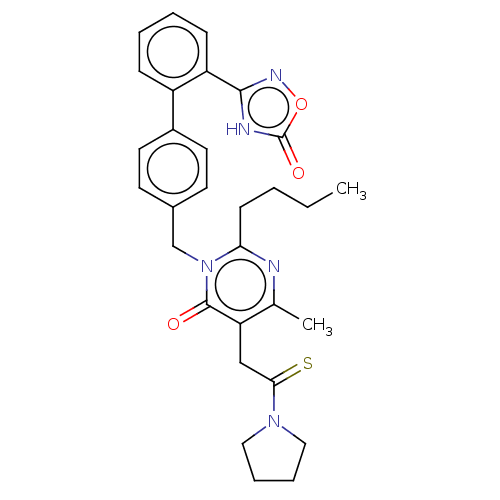
(CHEMBL4278280)Show SMILES CCCCc1nc(C)c(CC(=S)N2CCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H33N5O3S/c1-3-4-11-26-31-20(2)25(18-27(39)34-16-7-8-17-34)29(36)35(26)19-21-12-14-22(15-13-21)23-9-5-6-10-24(23)28-32-30(37)38-33-28/h5-6,9-10,12-15H,3-4,7-8,11,16-19H2,1-2H3,(H,32,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467130

(CHEMBL4291216)Show SMILES CCCCc1nc(C)c(CC(=S)N2CCCC2C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C31H35N5O3S/c1-4-5-12-27-32-21(3)26(18-28(40)35-17-8-9-20(35)2)30(37)36(27)19-22-13-15-23(16-14-22)24-10-6-7-11-25(24)29-33-31(38)39-34-29/h6-7,10-11,13-16,20H,4-5,8-9,12,17-19H2,1-3H3,(H,33,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50508111
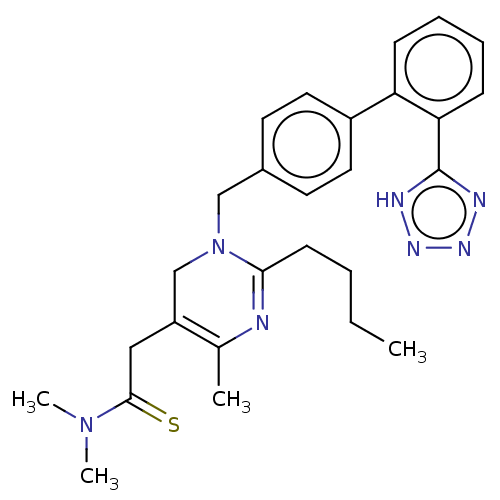
(CHEMBL4520827)Show SMILES CCCCC1=NC(C)=C(CC(=S)N(C)C)CN1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4,7| Show InChI InChI=1S/C27H33N7S/c1-5-6-11-25-28-19(2)22(16-26(35)33(3)4)18-34(25)17-20-12-14-21(15-13-20)23-9-7-8-10-24(23)27-29-31-32-30-27/h7-10,12-15H,5-6,11,16-18H2,1-4H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467140
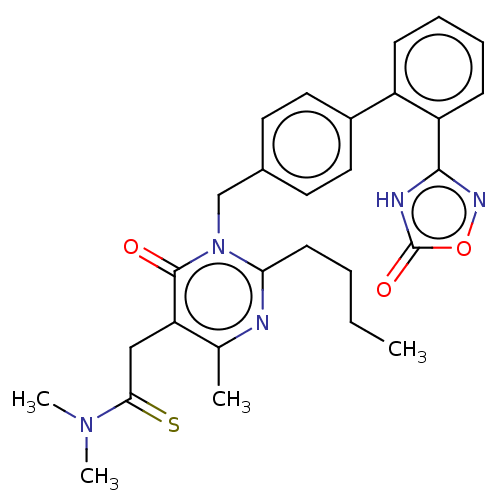
(CHEMBL4289788)Show SMILES CCCCc1nc(C)c(CC(=S)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C28H31N5O3S/c1-5-6-11-24-29-18(2)23(16-25(37)32(3)4)27(34)33(24)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)26-30-28(35)36-31-26/h7-10,12-15H,5-6,11,16-17H2,1-4H3,(H,30,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467133

(CHEMBL4280574)Show SMILES CCCCc1nc(C)c(CC(=S)N2[C@H](C)CC[C@H]2C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 |r| Show InChI InChI=1S/C32H37N5O3S/c1-5-6-11-28-33-22(4)27(18-29(41)37-20(2)12-13-21(37)3)31(38)36(28)19-23-14-16-24(17-15-23)25-9-7-8-10-26(25)30-34-32(39)40-35-30/h7-10,14-17,20-21H,5-6,11-13,18-19H2,1-4H3,(H,34,35,39)/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467135

(CHEMBL4287379)Show SMILES CCCCc1nc(C)c(CC(=S)N(CC)CC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H35N5O3S/c1-5-8-13-26-31-20(4)25(18-27(39)34(6-2)7-3)29(36)35(26)19-21-14-16-22(17-15-21)23-11-9-10-12-24(23)28-32-30(37)38-33-28/h9-12,14-17H,5-8,13,18-19H2,1-4H3,(H,32,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467138

(CHEMBL4282931)Show SMILES CCCCc1nc(C)c(CC(=S)N(C)CC(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C31H37N5O3S/c1-6-7-12-27-32-21(4)26(17-28(40)35(5)18-20(2)3)30(37)36(27)19-22-13-15-23(16-14-22)24-10-8-9-11-25(24)29-33-31(38)39-34-29/h8-11,13-16,20H,6-7,12,17-19H2,1-5H3,(H,33,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467131

(CHEMBL4288964)Show SMILES CCCCc1nc(C(C)C)c(CC(=S)N2CCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C32H37N5O3S/c1-4-5-12-27-33-29(21(2)3)26(19-28(41)36-17-8-9-18-36)31(38)37(27)20-22-13-15-23(16-14-22)24-10-6-7-11-25(24)30-34-32(39)40-35-30/h6-7,10-11,13-16,21H,4-5,8-9,12,17-20H2,1-3H3,(H,34,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50508110

(CHEMBL4444760)Show SMILES CCCCC1=NC(C(C)C)=C(CC(=S)N2CCCC2)CN1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 |t:4,9| Show InChI InChI=1S/C32H39N5O2S/c1-4-5-12-28-33-30(22(2)3)25(19-29(40)36-17-8-9-18-36)21-37(28)20-23-13-15-24(16-14-23)26-10-6-7-11-27(26)31-34-32(38)39-35-31/h6-7,10-11,13-16,22H,4-5,8-9,12,17-21H2,1-3H3,(H,34,35,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50364573
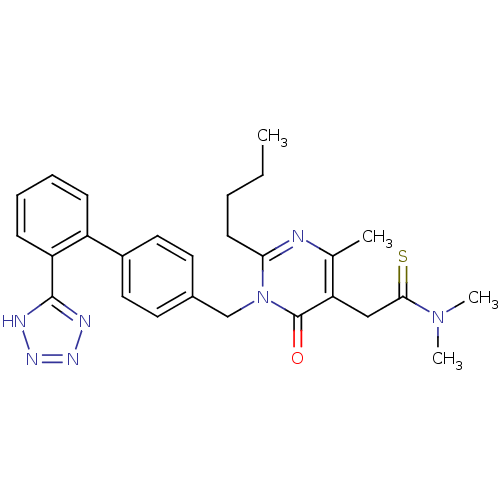
(CHEMBL1951143)Show SMILES CCCCc1nc(C)c(CC(=S)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H31N7OS/c1-5-6-11-24-28-18(2)23(16-25(36)33(3)4)27(35)34(24)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)26-29-31-32-30-26/h7-10,12-15H,5-6,11,16-17H2,1-4H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467139

(CHEMBL4293170)Show SMILES CCCc1nc(C)c(CC(=S)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C27H29N5O3S/c1-5-8-23-28-17(2)22(15-24(36)31(3)4)26(33)32(23)16-18-11-13-19(14-12-18)20-9-6-7-10-21(20)25-29-27(34)35-30-25/h6-7,9-14H,5,8,15-16H2,1-4H3,(H,29,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467136
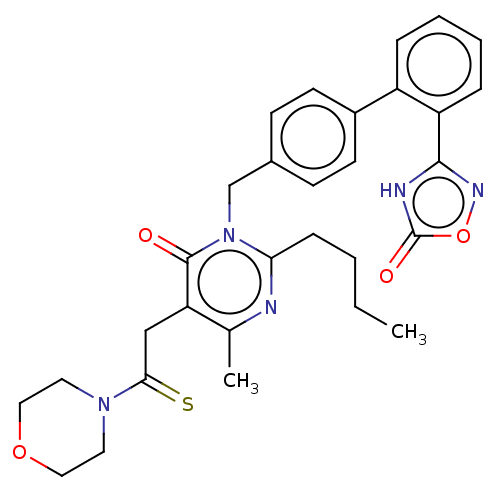
(CHEMBL4284022)Show SMILES CCCCc1nc(C)c(CC(=S)N2CCOCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H33N5O4S/c1-3-4-9-26-31-20(2)25(18-27(40)34-14-16-38-17-15-34)29(36)35(26)19-21-10-12-22(13-11-21)23-7-5-6-8-24(23)28-32-30(37)39-33-28/h5-8,10-13H,3-4,9,14-19H2,1-2H3,(H,32,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467131

(CHEMBL4288964)Show SMILES CCCCc1nc(C(C)C)c(CC(=S)N2CCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C32H37N5O3S/c1-4-5-12-27-33-29(21(2)3)26(19-28(41)36-17-8-9-18-36)31(38)37(27)20-22-13-15-23(16-14-22)24-10-6-7-11-25(24)30-34-32(39)40-35-30/h6-7,10-11,13-16,21H,4-5,8-9,12,17-20H2,1-3H3,(H,34,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by beta-arrestin recruitment assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467134
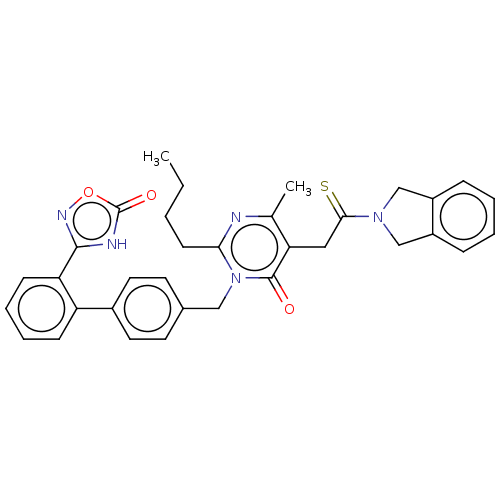
(CHEMBL4284403)Show SMILES CCCCc1nc(C)c(CC(=S)N2Cc3ccccc3C2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C34H33N5O3S/c1-3-4-13-30-35-22(2)29(18-31(43)38-20-25-9-5-6-10-26(25)21-38)33(40)39(30)19-23-14-16-24(17-15-23)27-11-7-8-12-28(27)32-36-34(41)42-37-32/h5-12,14-17H,3-4,13,18-21H2,1-2H3,(H,36,37,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467137
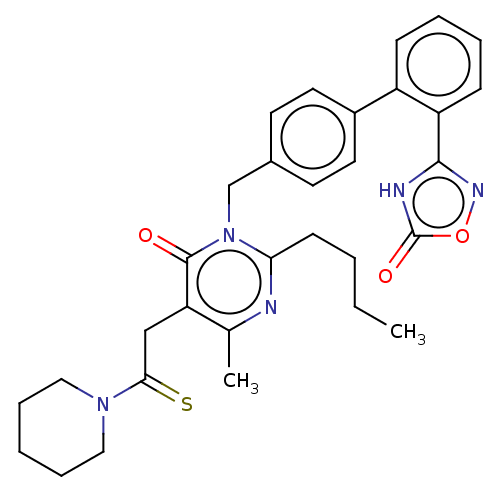
(CHEMBL4294618)Show SMILES CCCCc1nc(C)c(CC(=S)N2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C31H35N5O3S/c1-3-4-12-27-32-21(2)26(19-28(40)35-17-8-5-9-18-35)30(37)36(27)20-22-13-15-23(16-14-22)24-10-6-7-11-25(24)29-33-31(38)39-34-29/h6-7,10-11,13-16H,3-5,8-9,12,17-20H2,1-2H3,(H,33,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50467132

(CHEMBL4292297)Show SMILES [H][C@]12CCN(C(=S)Cc3c(C)nc(CCCC)n(Cc4ccc(cc4)-c4ccccc4-c4noc(=O)[nH]4)c3=O)[C@]1([H])CCCC2 |r| Show InChI InChI=1S/C34H39N5O3S/c1-3-4-13-30-35-22(2)28(20-31(43)38-19-18-25-9-5-8-12-29(25)38)33(40)39(30)21-23-14-16-24(17-15-23)26-10-6-7-11-27(26)32-36-34(41)42-37-32/h6-7,10-11,14-17,25,29H,3-5,8-9,12-13,18-21H2,1-2H3,(H,36,37,41)/t25-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at type-1 angiotensin 2 receptor (unknown origin) by calcium mobilizing assay |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508112

(CHEMBL4546437)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H34N4O5/c1-5-7-12-25-31-27(19(3)4)24(17-26(35)38-6-2)29(36)34(25)18-20-13-15-21(16-14-20)22-10-8-9-11-23(22)28-32-30(37)39-33-28/h8-11,13-16,19H,5-7,12,17-18H2,1-4H3,(H,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) by TR-FRET competitive binding assay |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50467131

(CHEMBL4288964)Show SMILES CCCCc1nc(C(C)C)c(CC(=S)N2CCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C32H37N5O3S/c1-4-5-12-27-33-29(21(2)3)26(19-28(41)36-17-8-9-18-36)31(38)37(27)20-22-13-15-23(16-14-22)24-10-6-7-11-25(24)30-34-32(39)40-35-30/h6-7,10-11,13-16,21H,4-5,8-9,12,17-20H2,1-3H3,(H,34,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 28: 3155-3160 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.036
BindingDB Entry DOI: 10.7270/Q2X63QMZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50508112

(CHEMBL4546437)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H34N4O5/c1-5-7-12-25-31-27(19(3)4)24(17-26(35)38-6-2)29(36)34(25)18-20-13-15-21(16-14-20)22-10-8-9-11-23(22)28-32-30(37)39-33-28/h8-11,13-16,19H,5-7,12,17-18H2,1-4H3,(H,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by automated patch clamp method |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50508112

(CHEMBL4546437)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H34N4O5/c1-5-7-12-25-31-27(19(3)4)24(17-26(35)38-6-2)29(36)34(25)18-20-13-15-21(16-14-20)22-10-8-9-11-23(22)28-32-30(37)39-33-28/h8-11,13-16,19H,5-7,12,17-18H2,1-4H3,(H,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PPARdelta (unknown origin) by TR-FRET competitive binding assay |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50508112

(CHEMBL4546437)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H34N4O5/c1-5-7-12-25-31-27(19(3)4)24(17-26(35)38-6-2)29(36)34(25)18-20-13-15-21(16-14-20)22-10-8-9-11-23(22)28-32-30(37)39-33-28/h8-11,13-16,19H,5-7,12,17-18H2,1-4H3,(H,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) by TR-FRET competitive binding assay |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508122

(CHEMBL4456647)Show SMILES CCCCc1nc(C)c(CC(=O)OC2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C32H36N4O5/c1-3-4-14-28-33-21(2)27(19-29(37)40-24-10-6-5-7-11-24)31(38)36(28)20-22-15-17-23(18-16-22)25-12-8-9-13-26(25)30-34-32(39)41-35-30/h8-9,12-13,15-18,24H,3-7,10-11,14,19-20H2,1-2H3,(H,34,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508123
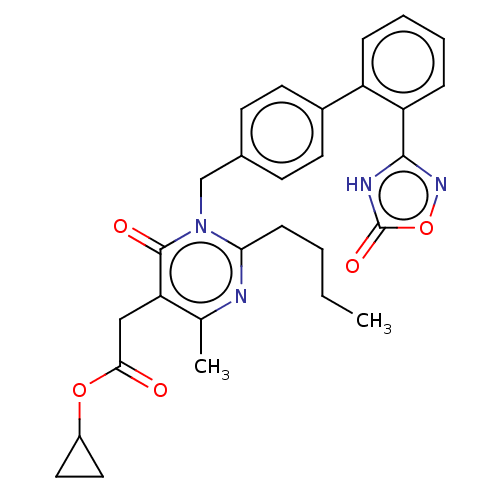
(CHEMBL4452865)Show SMILES CCCCc1nc(C)c(CC(=O)OC2CC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C29H30N4O5/c1-3-4-9-25-30-18(2)24(16-26(34)37-21-14-15-21)28(35)33(25)17-19-10-12-20(13-11-19)22-7-5-6-8-23(22)27-31-29(36)38-32-27/h5-8,10-13,21H,3-4,9,14-17H2,1-2H3,(H,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508112

(CHEMBL4546437)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H34N4O5/c1-5-7-12-25-31-27(19(3)4)24(17-26(35)38-6-2)29(36)34(25)18-20-13-15-21(16-14-20)22-10-8-9-11-23(22)28-32-30(37)39-33-28/h8-11,13-16,19H,5-7,12,17-18H2,1-4H3,(H,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508124
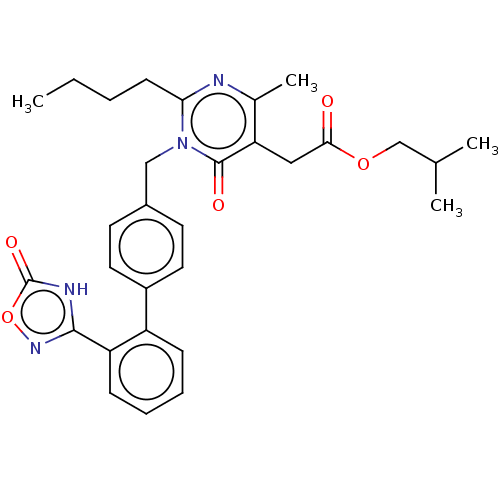
(CHEMBL4443588)Show SMILES CCCCc1nc(C)c(CC(=O)OCC(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H34N4O5/c1-5-6-11-26-31-20(4)25(16-27(35)38-18-19(2)3)29(36)34(26)17-21-12-14-22(15-13-21)23-9-7-8-10-24(23)28-32-30(37)39-33-28/h7-10,12-15,19H,5-6,11,16-18H2,1-4H3,(H,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535728
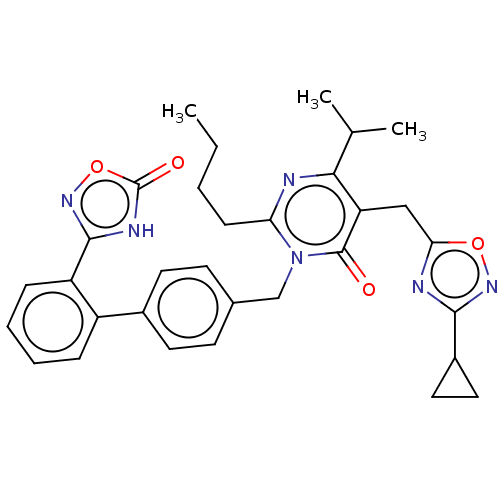
(CHEMBL4556396)Show SMILES CCCCc1nc(C(C)C)c(Cc2nc(no2)C2CC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C32H34N6O4/c1-4-5-10-26-33-28(19(2)3)25(17-27-34-29(36-41-27)22-15-16-22)31(39)38(26)18-20-11-13-21(14-12-20)23-8-6-7-9-24(23)30-35-32(40)42-37-30/h6-9,11-14,19,22H,4-5,10,15-18H2,1-3H3,(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535729

(CHEMBL4541559)Show SMILES CCCCc1nc(C)c(Cc2nc(COC)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C29H30N6O5/c1-4-5-10-25-30-18(2)23(15-26-31-24(17-38-3)33-39-26)28(36)35(25)16-19-11-13-20(14-12-19)21-8-6-7-9-22(21)27-32-29(37)40-34-27/h6-9,11-14H,4-5,10,15-17H2,1-3H3,(H,32,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535730
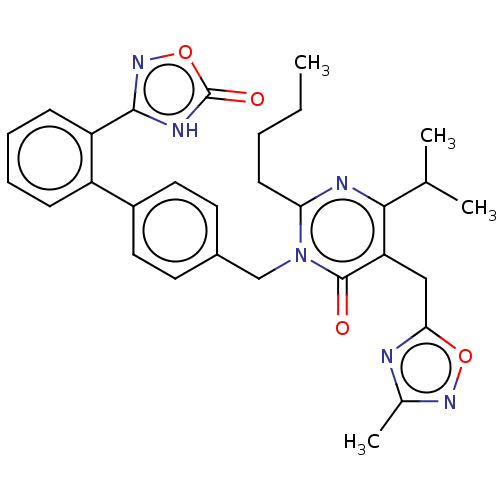
(CHEMBL4550608)Show SMILES CCCCc1nc(C(C)C)c(Cc2nc(C)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H32N6O4/c1-5-6-11-25-32-27(18(2)3)24(16-26-31-19(4)34-39-26)29(37)36(25)17-20-12-14-21(15-13-20)22-9-7-8-10-23(22)28-33-30(38)40-35-28/h7-10,12-15,18H,5-6,11,16-17H2,1-4H3,(H,33,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535731

(CHEMBL4435259)Show SMILES CCCCc1nc(C)c(Cc2nc(no2)C2CC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H30N6O4/c1-3-4-9-25-31-18(2)24(16-26-32-27(34-39-26)21-14-15-21)29(37)36(25)17-19-10-12-20(13-11-19)22-7-5-6-8-23(22)28-33-30(38)40-35-28/h5-8,10-13,21H,3-4,9,14-17H2,1-2H3,(H,33,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.25E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535732

(CHEMBL4448988)Show SMILES CCCCc1nc(C)c(Cc2nc(Cc3ccc(F)cc3)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C34H31FN6O4/c1-3-4-9-30-36-21(2)28(19-31-37-29(39-44-31)18-22-12-16-25(35)17-13-22)33(42)41(30)20-23-10-14-24(15-11-23)26-7-5-6-8-27(26)32-38-34(43)45-40-32/h5-8,10-17H,3-4,9,18-20H2,1-2H3,(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535733

(CHEMBL4447835)Show SMILES CCCCc1nc(C(C)C)c(Cc2nc(Cc3ccc(F)cc3)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C36H35FN6O4/c1-4-5-10-31-39-33(22(2)3)29(20-32-38-30(41-46-32)19-23-13-17-26(37)18-14-23)35(44)43(31)21-24-11-15-25(16-12-24)27-8-6-7-9-28(27)34-40-36(45)47-42-34/h6-9,11-18,22H,4-5,10,19-21H2,1-3H3,(H,40,42,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535734

(CHEMBL4444888)Show SMILES CCCCc1nc(C)c(Cc2nc(no2)C(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H32N6O4/c1-5-6-11-25-31-19(4)24(16-26-32-27(18(2)3)34-39-26)29(37)36(25)17-20-12-14-21(15-13-20)22-9-7-8-10-23(22)28-33-30(38)40-35-28/h7-10,12-15,18H,5-6,11,16-17H2,1-4H3,(H,33,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535735
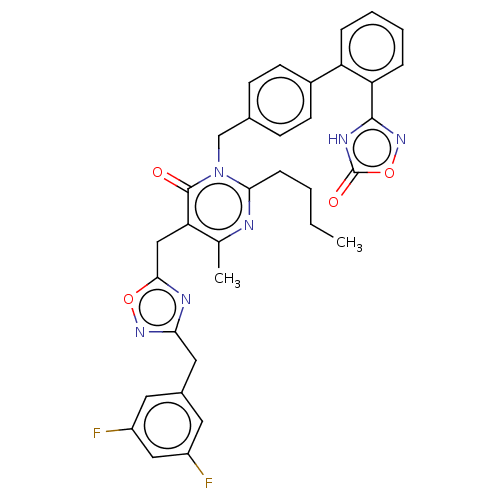
(CHEMBL4440354)Show SMILES CCCCc1nc(C)c(Cc2nc(Cc3cc(F)cc(F)c3)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C34H30F2N6O4/c1-3-4-9-30-37-20(2)28(18-31-38-29(40-45-31)16-22-14-24(35)17-25(36)15-22)33(43)42(30)19-21-10-12-23(13-11-21)26-7-5-6-8-27(26)32-39-34(44)46-41-32/h5-8,10-15,17H,3-4,9,16,18-19H2,1-2H3,(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535736

(CHEMBL4468084)Show SMILES CCCCc1nc(C)c(Cc2nc(CCOC)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H32N6O5/c1-4-5-10-26-31-19(2)24(17-27-32-25(34-40-27)15-16-39-3)29(37)36(26)18-20-11-13-21(14-12-20)22-8-6-7-9-23(22)28-33-30(38)41-35-28/h6-9,11-14H,4-5,10,15-18H2,1-3H3,(H,33,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.21E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535737

(CHEMBL4546069)Show SMILES CCCCc1nc(C)c(Cc2nc(no2)C(C)(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C31H34N6O4/c1-6-7-12-25-32-19(2)24(17-26-33-29(36-40-26)31(3,4)5)28(38)37(25)18-20-13-15-21(16-14-20)22-10-8-9-11-23(22)27-34-30(39)41-35-27/h8-11,13-16H,6-7,12,17-18H2,1-5H3,(H,34,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535738

(CHEMBL4463957)Show SMILES CCCCc1nc(C(C)C)c(Cc2nc(Cc3ccccc3)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C36H36N6O4/c1-4-5-15-31-38-33(23(2)3)29(21-32-37-30(40-45-32)20-24-11-7-6-8-12-24)35(43)42(31)22-25-16-18-26(19-17-25)27-13-9-10-14-28(27)34-39-36(44)46-41-34/h6-14,16-19,23H,4-5,15,20-22H2,1-3H3,(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535739

(CHEMBL4468637)Show SMILES CCCCc1nc(C)c(Cc2nc(Cc3cccc(F)c3)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C34H31FN6O4/c1-3-4-12-30-36-21(2)28(19-31-37-29(39-44-31)18-23-8-7-9-25(35)17-23)33(42)41(30)20-22-13-15-24(16-14-22)26-10-5-6-11-27(26)32-38-34(43)45-40-32/h5-11,13-17H,3-4,12,18-20H2,1-2H3,(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535740
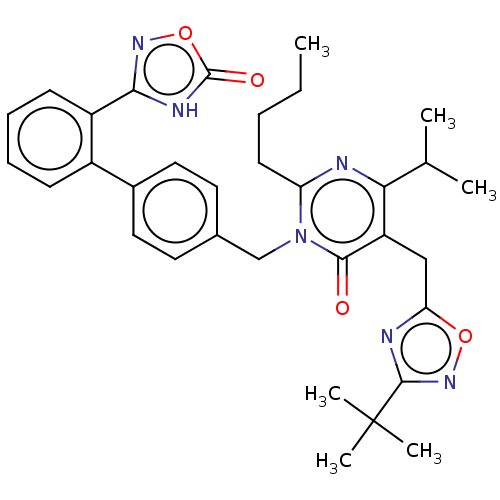
(CHEMBL4465311)Show SMILES CCCCc1nc(C(C)C)c(Cc2nc(no2)C(C)(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C33H38N6O4/c1-7-8-13-26-34-28(20(2)3)25(18-27-35-31(38-42-27)33(4,5)6)30(40)39(26)19-21-14-16-22(17-15-21)23-11-9-10-12-24(23)29-36-32(41)43-37-29/h9-12,14-17,20H,7-8,13,18-19H2,1-6H3,(H,36,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508121
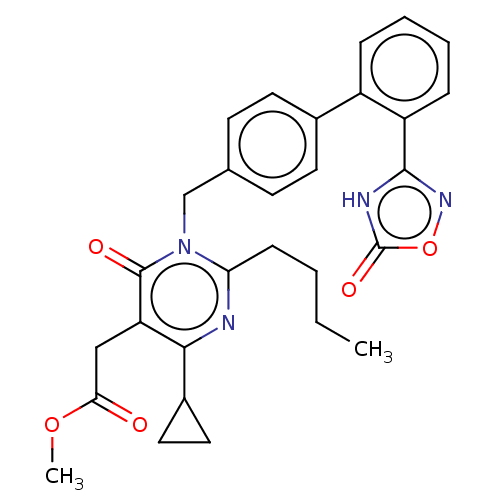
(CHEMBL4527893)Show SMILES CCCCc1nc(C2CC2)c(CC(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C29H30N4O5/c1-3-4-9-24-30-26(20-14-15-20)23(16-25(34)37-2)28(35)33(24)17-18-10-12-19(13-11-18)21-7-5-6-8-22(21)27-31-29(36)38-32-27/h5-8,10-13,20H,3-4,9,14-17H2,1-2H3,(H,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508120

(CHEMBL4531918)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C29H32N4O5/c1-5-6-11-24-30-26(18(2)3)23(16-25(34)37-4)28(35)33(24)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)27-31-29(36)38-32-27/h7-10,12-15,18H,5-6,11,16-17H2,1-4H3,(H,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508119

(CHEMBL4472191)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C32H38N4O5/c1-6-7-12-27-33-29(21(4)5)26(17-28(37)40-19-20(2)3)31(38)36(27)18-22-13-15-23(16-14-22)24-10-8-9-11-25(24)30-34-32(39)41-35-30/h8-11,13-16,20-21H,6-7,12,17-19H2,1-5H3,(H,34,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508118
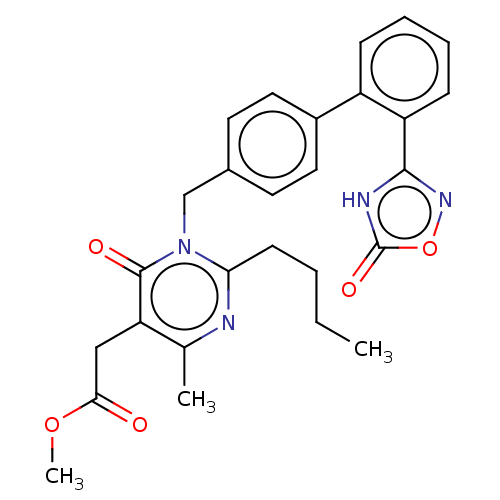
(CHEMBL4436393)Show SMILES CCCCc1nc(C)c(CC(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C27H28N4O5/c1-4-5-10-23-28-17(2)22(15-24(32)35-3)26(33)31(23)16-18-11-13-19(14-12-18)20-8-6-7-9-21(20)25-29-27(34)36-30-25/h6-9,11-14H,4-5,10,15-16H2,1-3H3,(H,29,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.36E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508117
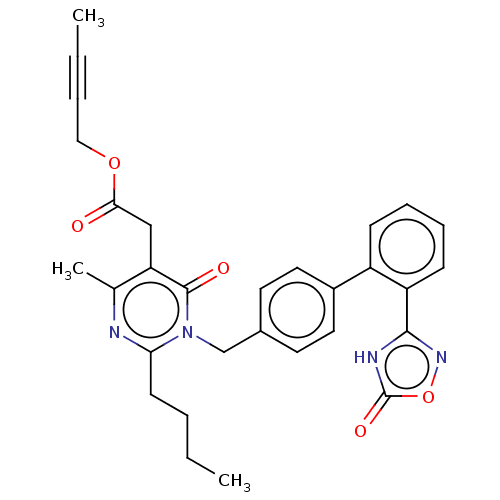
(CHEMBL4463602)Show SMILES CCCCc1nc(C)c(CC(=O)OCC#CC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H30N4O5/c1-4-6-12-26-31-20(3)25(18-27(35)38-17-7-5-2)29(36)34(26)19-21-13-15-22(16-14-21)23-10-8-9-11-24(23)28-32-30(37)39-33-28/h8-11,13-16H,4,6,12,17-19H2,1-3H3,(H,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508116

(CHEMBL4535401)Show SMILES CCCCc1nc(C(C)C)c(CC(=O)OCC(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H31F3N4O5/c1-4-5-10-24-34-26(18(2)3)23(15-25(38)41-17-30(31,32)33)28(39)37(24)16-19-11-13-20(14-12-19)21-8-6-7-9-22(21)27-35-29(40)42-36-27/h6-9,11-14,18H,4-5,10,15-17H2,1-3H3,(H,35,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508115
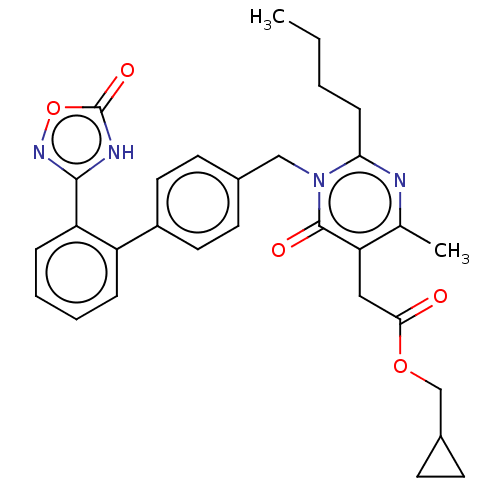
(CHEMBL4469751)Show SMILES CCCCc1nc(C)c(CC(=O)OCC2CC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C30H32N4O5/c1-3-4-9-26-31-19(2)25(16-27(35)38-18-21-10-11-21)29(36)34(26)17-20-12-14-22(15-13-20)23-7-5-6-8-24(23)28-32-30(37)39-33-28/h5-8,12-15,21H,3-4,9-11,16-18H2,1-2H3,(H,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508114

(CHEMBL4466241)Show SMILES CCCCc1nc(C)c(CC(=O)OCC2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C33H38N4O5/c1-3-4-14-29-34-22(2)28(19-30(38)41-21-24-10-6-5-7-11-24)32(39)37(29)20-23-15-17-25(18-16-23)26-12-8-9-13-27(26)31-35-33(40)42-36-31/h8-9,12-13,15-18,24H,3-7,10-11,14,19-21H2,1-2H3,(H,35,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508113

(CHEMBL4453360)Show SMILES CCCCc1nc(C2CC2)c(CC(=O)OC(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C31H34N4O5/c1-4-5-10-26-32-28(22-15-16-22)25(17-27(36)39-19(2)3)30(37)35(26)18-20-11-13-21(14-12-20)23-8-6-7-9-24(23)29-33-31(38)40-34-29/h6-9,11-14,19,22H,4-5,10,15-18H2,1-3H3,(H,33,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508111

(CHEMBL4520827)Show SMILES CCCCC1=NC(C)=C(CC(=S)N(C)C)CN1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4,7| Show InChI InChI=1S/C27H33N7S/c1-5-6-11-25-28-19(2)22(16-26(35)33(3)4)18-34(25)17-20-12-14-21(15-13-20)23-9-7-8-10-24(23)27-29-31-32-30-27/h7-10,12-15H,5-6,11,16-18H2,1-4H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50535741

(CHEMBL4464717)Show SMILES CCCCc1nc(C)c(Cc2nc(Cc3ccccc3)no2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C34H32N6O4/c1-3-4-14-30-35-22(2)28(20-31-36-29(38-43-31)19-23-10-6-5-7-11-23)33(41)40(30)21-24-15-17-25(18-16-24)26-12-8-9-13-27(26)32-37-34(42)44-39-32/h5-13,15-18H,3-4,14,19-21H2,1-2H3,(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) assessed as receptor transactivation after 24 hrs by TK-PPRE-Luc expressing cells based luciferase rep... |
Bioorg Med Chem Lett 29: 2275-2282 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.027
BindingDB Entry DOI: 10.7270/Q2RR22RG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50508109
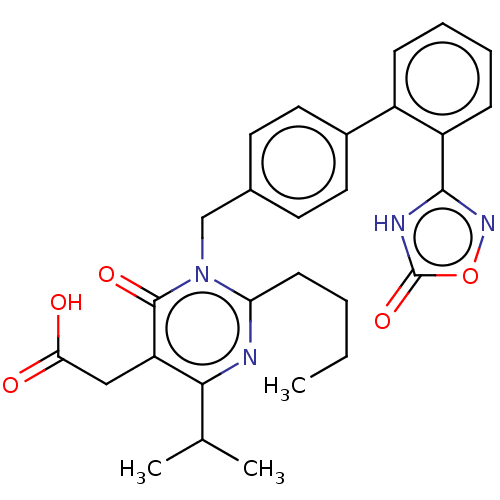
(CHEMBL4592935)Show SMILES CCCCc1nc(C(C)C)c(CC(O)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1noc(=O)[nH]1 Show InChI InChI=1S/C28H30N4O5/c1-4-5-10-23-29-25(17(2)3)22(15-24(33)34)27(35)32(23)16-18-11-13-19(14-12-18)20-8-6-7-9-21(20)26-30-28(36)37-31-26/h6-9,11-14,17H,4-5,10,15-16H2,1-3H3,(H,33,34)(H,30,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a |
Boryung Pharmaceuticals Co. Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem Lett 29: 631-637 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.043
BindingDB Entry DOI: 10.7270/Q22B929Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data