 Found 14337 hits with Last Name = 'wang' and Initial = 'w'
Found 14337 hits with Last Name = 'wang' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
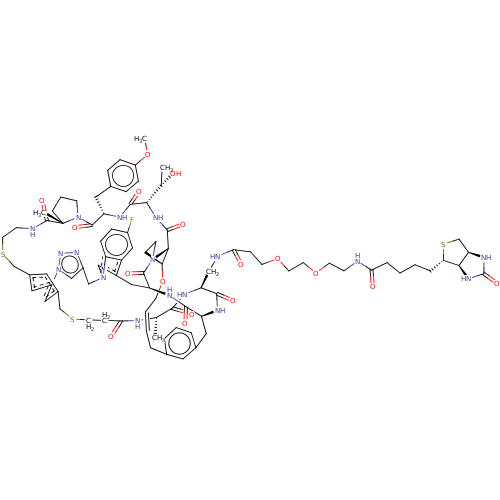
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
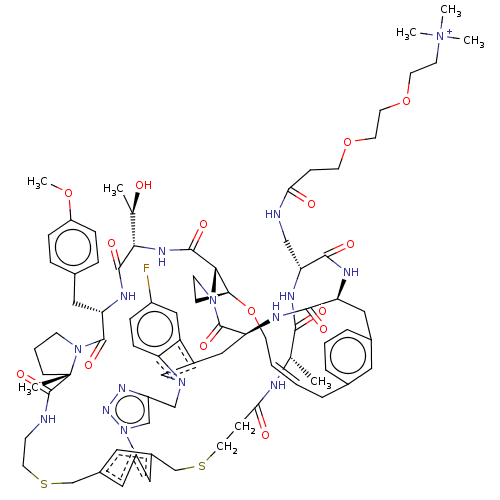
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
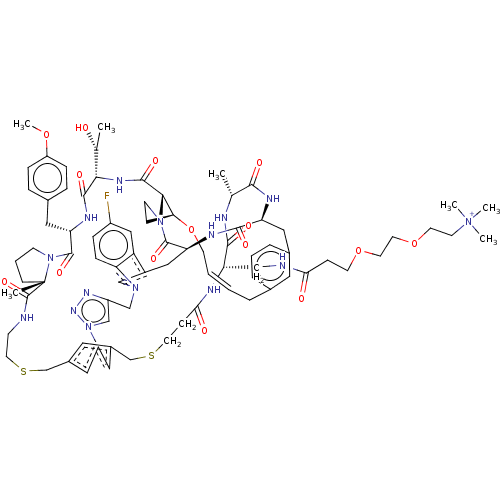
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
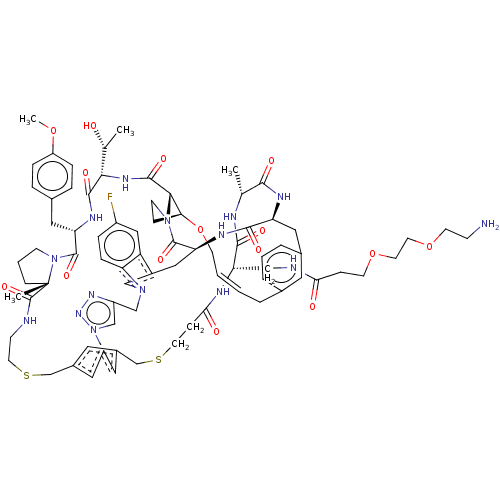
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
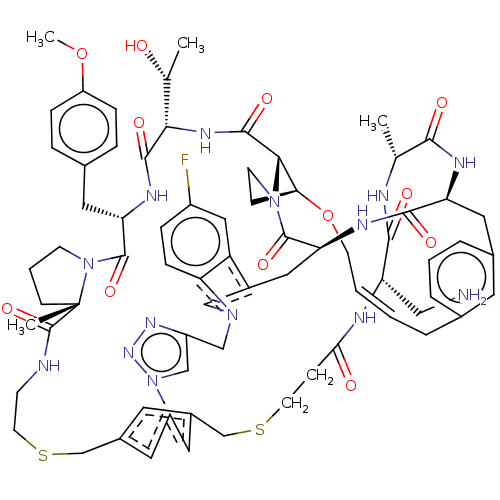
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598031

((R)-Tetrahydrofuran-3-yl(8-amino-7- | US11612606, ...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3CCOC3)ncc2c(N)c1F |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598056
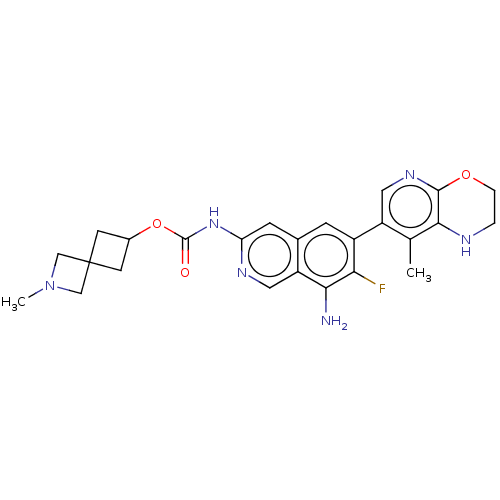
(2-Methyl-2-azaspiro[3.3]heptan-6-yl(8- amino-7-flu...)Show SMILES CN1CC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598057
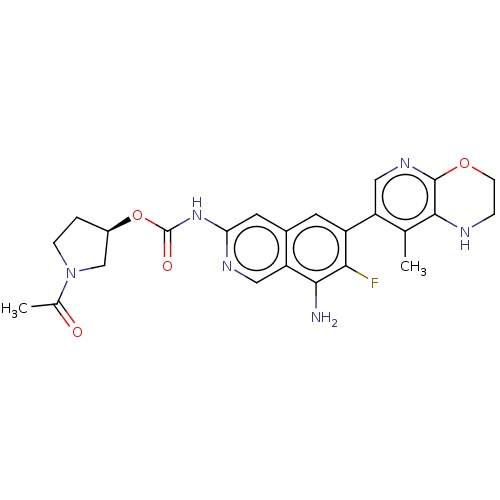
((R)-1-Acetylpyrrolidin-3-yl(8-amino-7- | US1161260...)Show SMILES CC(=O)N1CC[C@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598059
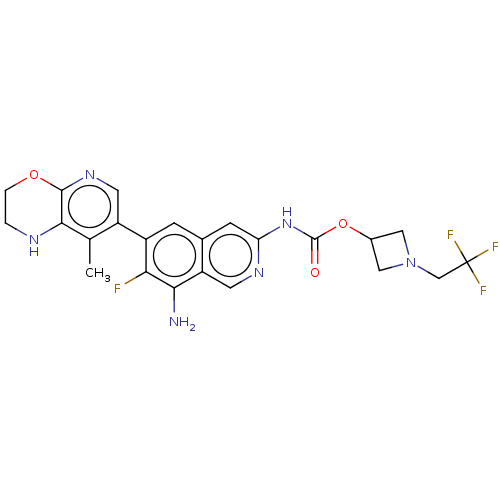
(1-(2,2,2-Trifluoroethyl)azetidin-3-yl(8- amino-7-f...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CN(CC(F)(F)F)C3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598060
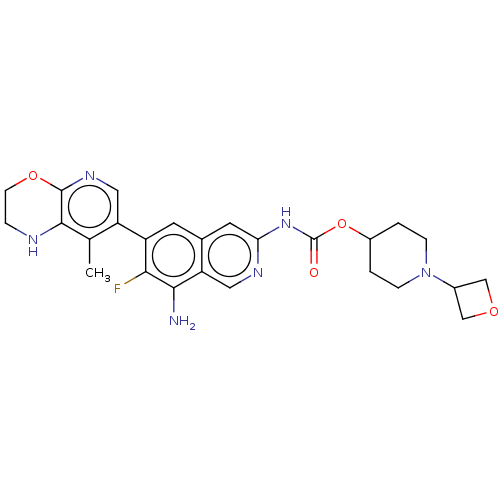
(1-(Oxetan-3-yl)piperidin-4-yl(8-amino- 7-fluoro-6-...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CCN(CC3)C3COC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598062

(7-Methyl-7-azaspiro[3.5]nonan-2-yl(8- amino-7-fluo...)Show SMILES CN1CCC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3CCCNc3c2C)CC1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598063

(2-Acetyl-2-azaspiro[3.3]heptan-6-yl(8- amino-7-flu...)Show SMILES CC(=O)N1CC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598249

((1s,3s)-3-(Hydroxymethyl)cyclobutyl(8- amino-7-flu...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3C[C@H](CO)C3)ncc2c(N)c1F |r,wD:20.21,22.24,(2.55,-1.61,;3.88,-.84,;5.21,-1.61,;5.21,-3.15,;6.55,-3.92,;7.88,-3.15,;7.88,-1.61,;6.55,-.84,;6.55,.7,;5.21,1.47,;3.88,.7,;2.55,1.47,;1.21,.7,;-.12,1.47,;-1.46,.7,;-2.79,1.47,;-4.12,.7,;-5.46,1.47,;-5.46,3.01,;-6.79,.7,;-6.79,-.84,;-5.7,-1.93,;-6.79,-3.01,;-6.79,-4.55,;-5.46,-5.32,;-7.88,-1.93,;-2.79,3.01,;-1.46,3.78,;-.12,3.01,;1.21,3.78,;1.21,5.32,;2.55,3.01,;3.88,3.78,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598250
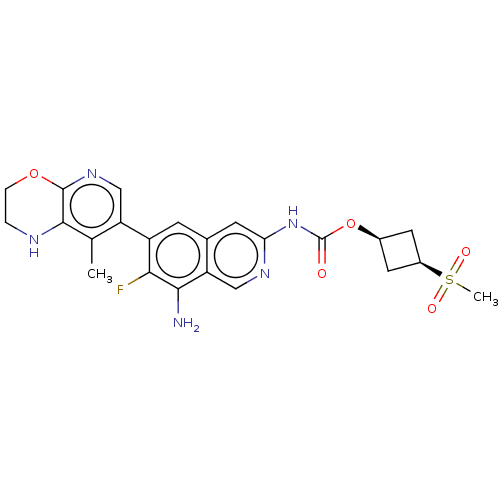
((1s,3s)-3-(Methylsulfonyl)cyclobutyl(8- | US116126...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@H](C3)S(C)(=O)=O)ncc2c(N)c1F |r,wU:20.21,22.26,(2.1,-.07,;3.44,.7,;4.77,-.07,;4.77,-1.61,;6.1,-2.38,;7.44,-1.61,;7.44,-.07,;6.1,.7,;6.1,2.24,;4.77,3.01,;3.44,2.24,;2.1,3.01,;.77,2.24,;-.56,3.01,;-1.9,2.24,;-3.23,3.01,;-4.56,2.24,;-4.56,.7,;-3.23,-.07,;-5.9,-.07,;-5.9,-1.61,;-4.81,-2.69,;-5.9,-3.78,;-6.99,-2.69,;-5.9,-5.32,;-5.9,-6.86,;-4.36,-5.32,;-7.44,-5.32,;-3.23,4.55,;-1.9,5.32,;-.56,4.55,;.77,5.32,;.77,6.86,;2.1,4.55,;3.44,5.32,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598254

((1R,3s)-3-((S)-1- Hydroxyethyl)cyclobutyl(8-amino-...)Show SMILES C[C@H](O)[C@H]1C[C@@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r,wU:3.2,1.1,wD:5.7,(-8,-5.32,;-6.67,-4.55,;-5.33,-5.32,;-6.67,-3.01,;-7.76,-1.93,;-6.67,-.84,;-5.58,-1.93,;-6.67,.7,;-5.33,1.47,;-5.33,3.01,;-4,.7,;-2.67,1.47,;-1.33,.7,;,1.47,;1.33,.7,;2.67,1.47,;2.67,3.01,;4,3.78,;1.33,3.78,;1.33,5.32,;,3.01,;-1.33,3.78,;-2.67,3.01,;4,.7,;5.33,1.47,;6.67,.7,;6.67,-.84,;8,-1.61,;8,-3.15,;6.67,-3.92,;5.33,-3.15,;5.33,-1.61,;4,-.84,;2.67,-1.61,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598256
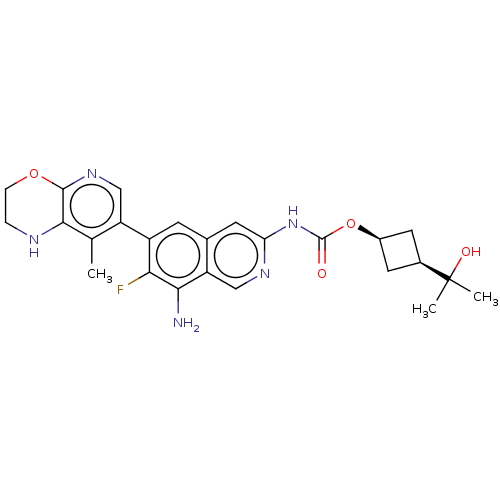
((1s,3s)-3-(2-Hydroxypropan-2- yl)cyclobutyl(8-amin...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@H](C3)C(C)(C)O)ncc2c(N)c1F |r,wD:20.21,22.26,(2.67,-1.22,;4,-.45,;5.33,-1.22,;5.33,-2.76,;6.67,-3.53,;8,-2.76,;8,-1.22,;6.67,-.45,;6.67,1.09,;5.33,1.86,;4,1.09,;2.67,1.86,;1.33,1.09,;,1.86,;-1.33,1.09,;-2.67,1.86,;-4,1.09,;-5.33,1.86,;-5.33,3.4,;-6.67,1.09,;-6.67,-.45,;-7.76,-1.54,;-6.67,-2.63,;-5.58,-1.54,;-6.67,-4.17,;-5.33,-4.94,;-8,-4.94,;-6.67,-5.71,;-2.67,3.4,;-1.33,4.17,;,3.4,;1.33,4.17,;1.33,5.71,;2.67,3.4,;4,4.17,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598257
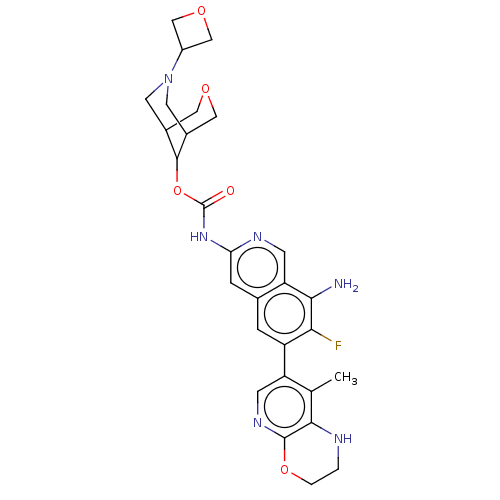
((1R,5S,9s)-7-(Oxetan-3-yl)-3-oxa-7- | US11612606, ...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3C4COCC3CN(C4)C3COC3)ncc2c(N)c1F |THB:29:27:20:22.24.23,19:20:28.27.26:22.24.23| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598258
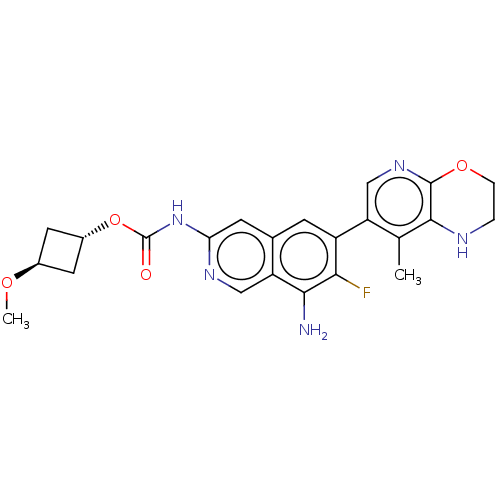
((1r,3r)-3-Methoxycyclobutyl(8-amino-7- | US1161260...)Show SMILES CO[C@H]1C[C@@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r,wU:4.6,wD:2.1,(-8,-5.32,;-6.67,-4.55,;-6.67,-3.01,;-5.58,-1.93,;-6.67,-.84,;-7.76,-1.93,;-6.67,.7,;-5.33,1.47,;-5.33,3.01,;-4,.7,;-2.67,1.47,;-1.33,.7,;,1.47,;1.33,.7,;2.67,1.47,;2.67,3.01,;4,3.78,;1.33,3.78,;1.33,5.32,;,3.01,;-1.33,3.78,;-2.67,3.01,;4,.7,;5.33,1.47,;6.67,.7,;6.67,-.84,;8,-1.61,;8,-3.15,;6.67,-3.92,;5.33,-3.15,;5.33,-1.61,;4,-.84,;2.67,-1.61,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598260

((1r,3r)-3-(Difluoromethyl)cyclobutyl(8- amino-7-fl...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@@H](C3)C(F)F)ncc2c(N)c1F |r,wU:20.21,wD:22.26,(2.67,-1.61,;4,-.84,;5.33,-1.61,;5.33,-3.15,;6.67,-3.92,;8,-3.15,;8,-1.61,;6.67,-.84,;6.67,.7,;5.33,1.47,;4,.7,;2.67,1.47,;1.33,.7,;,1.47,;-1.33,.7,;-2.67,1.47,;-4,.7,;-5.33,1.47,;-5.33,3.01,;-6.67,.7,;-6.67,-.84,;-5.58,-1.93,;-6.67,-3.01,;-7.76,-1.93,;-6.67,-4.55,;-8,-5.32,;-5.33,-5.32,;-2.67,3.01,;-1.33,3.78,;,3.01,;1.33,3.78,;1.33,5.32,;2.67,3.01,;4,3.78,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598264
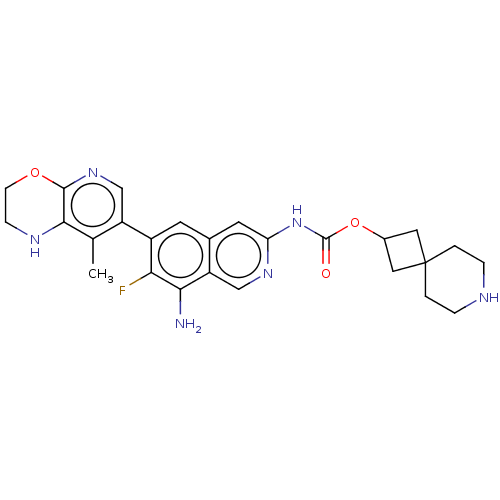
(7-Azaspiro[3.5]nonan-2-yl(8-amino-7- fluoro-6-(8-m...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CC4(C3)CCNCC4)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598064
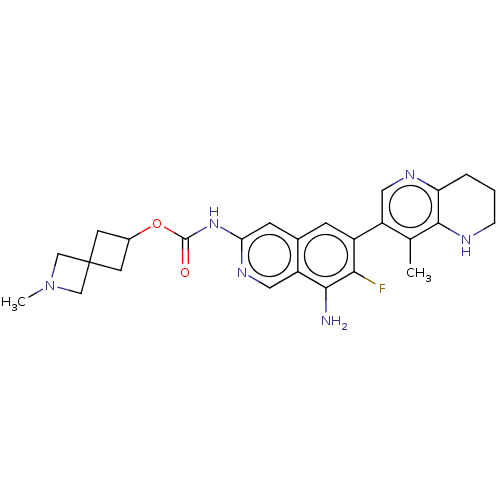
(2-Methyl-2-azaspiro[3.3]heptan-6-yl(8- amino-7-flu...)Show SMILES CN1CC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3CCCNc3c2C)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598065

(1-Acetylazetidin-3-yl(8-amino-7-fluoro- 6-(8-methy...)Show SMILES CC(=O)N1CC(C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598066

(7-Methyl-7-azaspiro[3.5]nonan-2-yl(8- amino-7-fluo...)Show SMILES CN1CCC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)CC1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598067
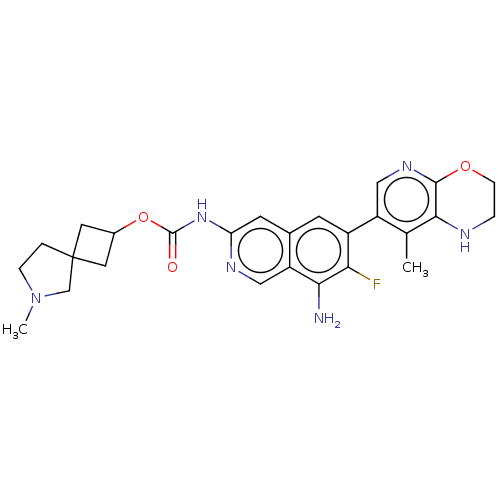
(6-Methyl-6-azaspiro[3.4]octan-2-yl(8- amino-7-fluo...)Show SMILES CN1CCC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 |(-5.17,-6.02,;-5.94,-4.69,;-7.48,-4.69,;-7.96,-3.22,;-6.71,-2.32,;-7.8,-1.23,;-6.71,-.14,;-5.62,-1.23,;-6.71,1.4,;-5.38,2.17,;-5.38,3.71,;-4.04,1.4,;-2.71,2.17,;-1.38,1.4,;-.04,2.17,;1.29,1.4,;2.62,2.17,;2.62,3.71,;3.96,4.48,;1.29,4.48,;1.29,6.02,;-.04,3.71,;-1.38,4.48,;-2.71,3.71,;3.96,1.4,;5.29,2.17,;6.62,1.4,;6.62,-.14,;7.96,-.91,;7.96,-2.45,;6.62,-3.22,;5.29,-2.45,;5.29,-.91,;3.96,-.14,;2.62,-.91,;-5.47,-3.22,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598069

(7-(Oxetan-3-yl)-7-azaspiro[3.5]nonan-2- yl(8-amino...)Show SMILES Cc1c2NCCCc2ncc1-c1cc2cc(NC(=O)OC3CC4(C3)CCN(CC4)C3COC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598071

((1R,5S,6r)-3-Methyl-3- | US11612606, Compound 432a)Show SMILES CN1C[C@H]2[C@@H](C1)[C@@H]2OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598072
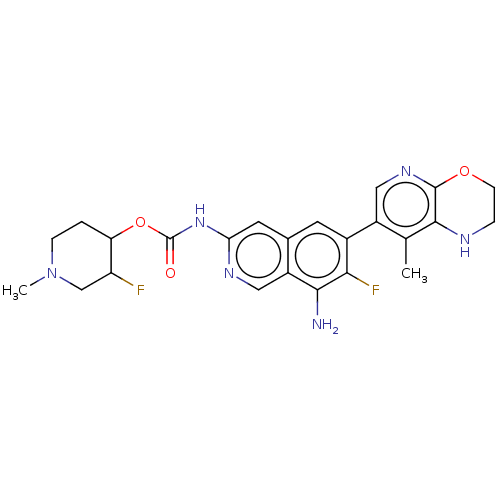
((cis)-3-Fluoro-1-methylpiperidin-4-yl(8- amino-7-f...)Show SMILES CN1CCC(OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C(F)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598073

(1-(2,2-Difluoroethypazetidin-3-yl(8- amino-7-fluor...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CN(CC(F)F)C3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598076

((3S,4R)-1-Acetyl-3-fluoropiperidin-4-yl | US116126...)Show SMILES CC(=O)N1CC[C@H](OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C(F)C1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598078

((1R,5S,9s)-7-Methyl-3-oxa-7- | US11612606, Compoun...)Show SMILES CN1CC2COC[C@H](C1)C2OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r,TLB:0:1:9:6.4.5,THB:10:9:6.4.5:2.8.1| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598082

((trans)-4-Methyltetrahydrofuran-3-yl(8- | US116126...)Show SMILES C[C@H]1COC[C@H]1OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598083
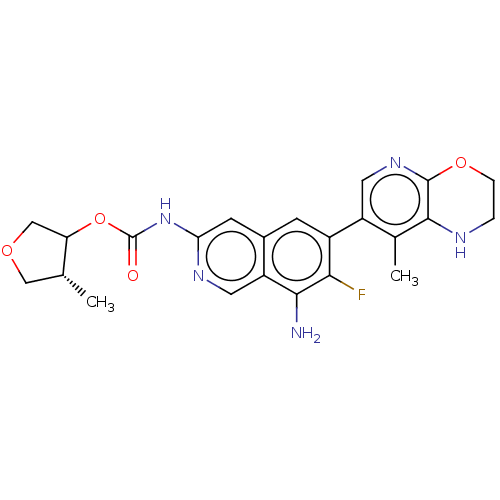
(US11612606, Compound 441b | amino-7-fluoro-6-(8-me...)Show SMILES C[C@@H]1COCC1OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598090
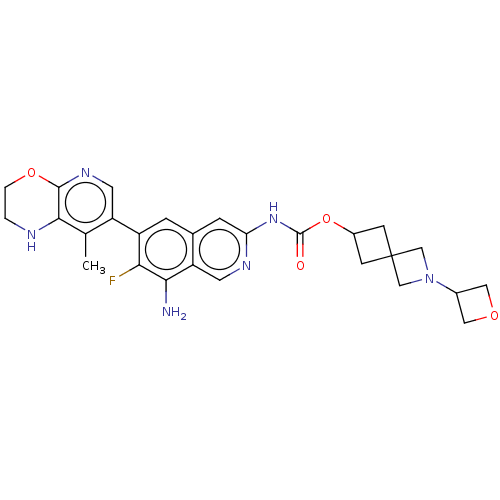
(2-(Oxetan-3-yl)-2-azaspiro[3.3]heptan-6- yl(8-amin...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CC4(C3)CN(C4)C3COC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598091

((S)-1-Methyl-5-oxopyrrolidin-3-yl(8- | US11612606,...)Show SMILES CCc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3CN(C)C(=O)C3)ncc2c(N)c1F |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598092

(US11612606, Compound 444b | amino-6-(8-ethyl-2,3-d...)Show SMILES CCc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3CN(C)C(=O)C3)ncc2c(N)c1F |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598093
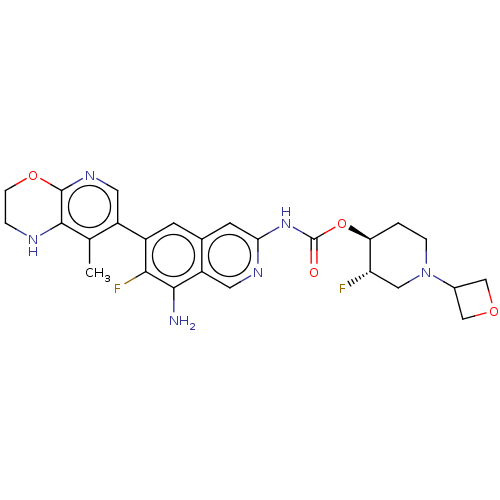
((+1-)-trans-3-Fluoro-1-(oxetan-3-yl)piperidin-4-yl...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3CCN(C[C@@H]3F)C3COC3)ncc2c(N)c1F |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598095
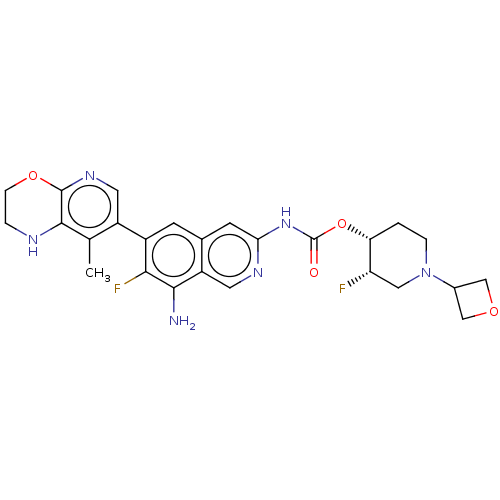
(US11612606, Compound 445d | yl(8-amino-7-fluoro-6-...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3CCN(C[C@@H]3F)C3COC3)ncc2c(N)c1F |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598096

((1r,3r)-3-(Dimethylamino)cyclobutyl(8- | US1161260...)Show SMILES CN(C)[C@H]1C[C@@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r,wU:5.7,wD:3.2,(-5.33,-5.32,;-6.67,-4.55,;-8,-5.32,;-6.67,-3.01,;-5.58,-1.93,;-6.67,-.84,;-7.76,-1.93,;-6.67,.7,;-5.33,1.47,;-5.33,3.01,;-4,.7,;-2.67,1.47,;-1.33,.7,;,1.47,;1.33,.7,;2.67,1.47,;2.67,3.01,;4,3.78,;1.33,3.78,;1.33,5.32,;,3.01,;-1.33,3.78,;-2.67,3.01,;4,.7,;5.33,1.47,;6.67,.7,;6.67,-.84,;8,-1.61,;8,-3.15,;6.67,-3.92,;5.33,-3.15,;5.33,-1.61,;4,-.84,;2.67,-1.61,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598099

(US11612606, Compound 451b | yl)methyl(8-amino-7-fl...)Show SMILES CN1CC[C@@](F)(COC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598308
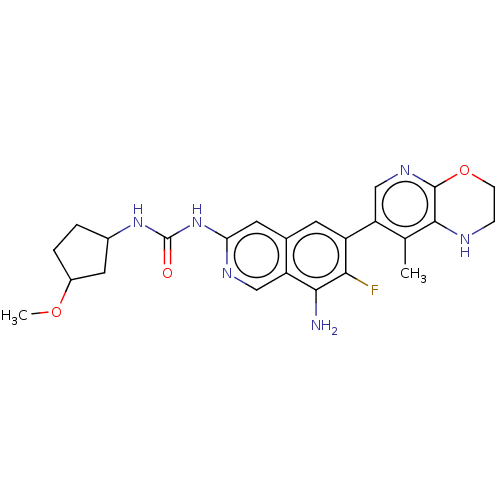
(1-(8-Amino-7-fluoro-6-(8-methyl-2,3-dihydro-1H-pyr...)Show SMILES COC1CCC(C1)NC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598309

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES CO[C@@H]1CC[C@H](C1)NC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598310

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES CO[C@H]1CC[C@@H](C1)NC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598311

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3-dihydro-1H-pyr...)Show SMILES CO[C@@H]1CC[C@@H](C1)NC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598312

(1-(8-Amino-7-fluoro-6-(4-methyl-5,6,7,8-tetrahydro...)Show SMILES Cc1c2NCCCc2ncc1-c1cc2cc(NC(=O)N[C@H]3C[C@H](F)C3)ncc2c(N)c1F |r,wU:20.21,wD:22.24,(1.88,-.84,;3.21,-.07,;4.55,-.84,;4.55,-2.38,;5.88,-3.15,;7.21,-2.38,;7.21,-.84,;5.88,-.07,;5.88,1.47,;4.55,2.24,;3.21,1.47,;1.88,2.24,;.54,1.47,;-.79,2.24,;-2.12,1.47,;-3.46,2.24,;-4.79,1.47,;-4.79,-.07,;-3.46,-.84,;-6.12,-.84,;-6.12,-2.38,;-5.03,-3.47,;-6.12,-4.55,;-6.12,-6.09,;-7.21,-3.47,;-3.46,3.78,;-2.12,4.55,;-.79,3.78,;.54,4.55,;.54,6.09,;1.88,3.78,;3.21,4.55,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data