

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579952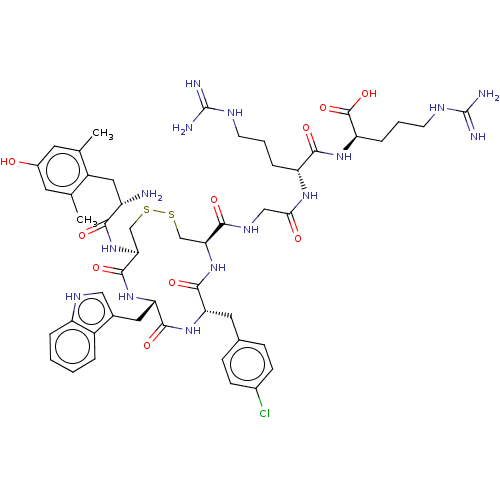 (CHEMBL5076581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579953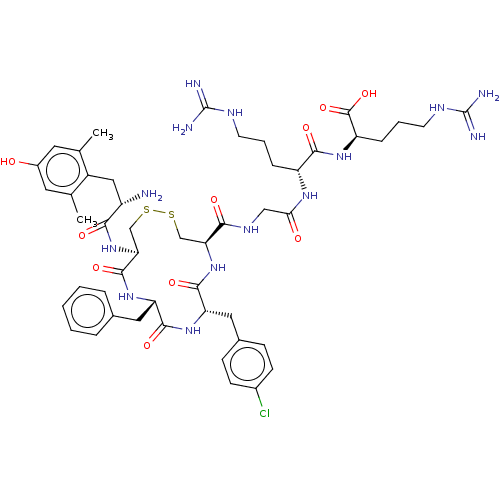 (CHEMBL5085104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579948 (CHEMBL5080666) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579949 (CHEMBL5084034) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579951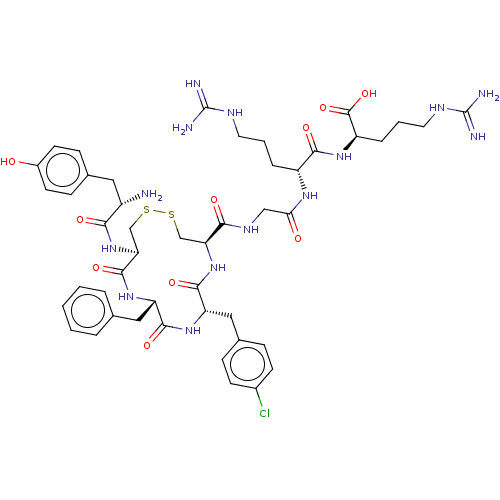 (CHEMBL5078349) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579950 (CHEMBL5080233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579952 (CHEMBL5076581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579952 (CHEMBL5076581) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579953 (CHEMBL5085104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579953 (CHEMBL5085104) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579950 (CHEMBL5080233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579951 (CHEMBL5078349) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579949 (CHEMBL5084034) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579948 (CHEMBL5080666) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579948 (CHEMBL5080666) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579949 (CHEMBL5084034) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579950 (CHEMBL5080233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50579951 (CHEMBL5078349) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus 1) | BDBM50452548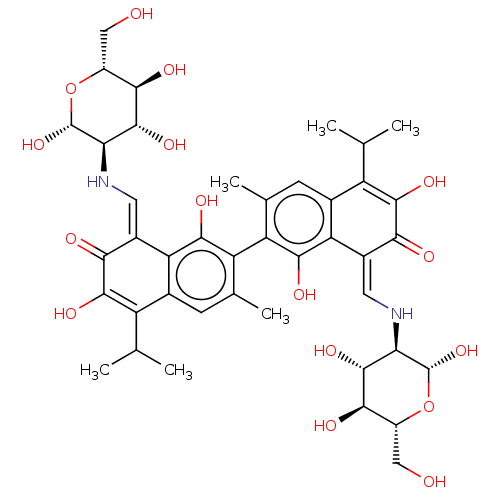 (CHEMBL2375867) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Renmin Hospital of Wuhan University Curated by ChEMBL | Assay Description Inhibition of HIV1 3B gp41-induced cell-cell fusion between viral protein expressing human H9 cells and MT-2 target cells assessed as number of syncy... | Bioorg Med Chem Lett 28: 49-52 (2018) Article DOI: 10.1016/j.bmcl.2017.08.049 BindingDB Entry DOI: 10.7270/Q2FX7D2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus 1) | BDBM50452548 (CHEMBL2375867) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Renmin Hospital of Wuhan University Curated by ChEMBL | Assay Description Inhibition of HIV gp41 6-helix bundle formation preincubated with C34 and N36 for 30 mins | Bioorg Med Chem Lett 28: 49-52 (2018) Article DOI: 10.1016/j.bmcl.2017.08.049 BindingDB Entry DOI: 10.7270/Q2FX7D2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus 1) | BDBM50452550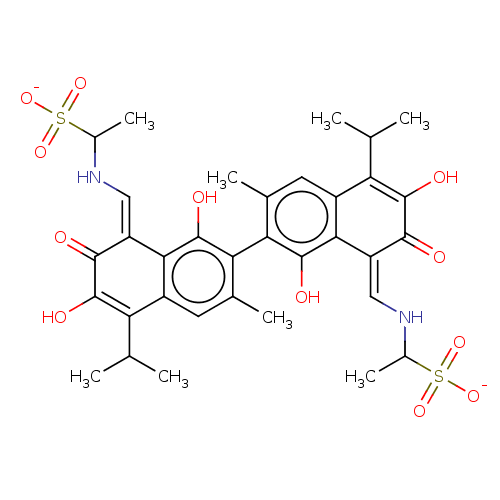 (CHEMBL4207660) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Renmin Hospital of Wuhan University Curated by ChEMBL | Assay Description Inhibition of HIV1 3B gp41-induced cell-cell fusion between viral protein expressing human H9 cells and MT-2 target cells assessed as number of syncy... | Bioorg Med Chem Lett 28: 49-52 (2018) Article DOI: 10.1016/j.bmcl.2017.08.049 BindingDB Entry DOI: 10.7270/Q2FX7D2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus 1) | BDBM50452549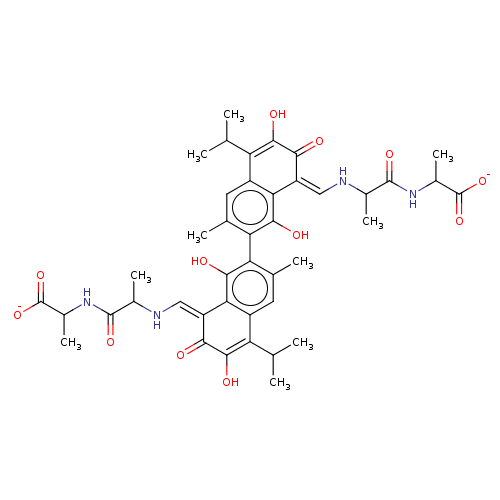 (CHEMBL4203285) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Renmin Hospital of Wuhan University Curated by ChEMBL | Assay Description Inhibition of HIV1 3B gp41-induced cell-cell fusion between viral protein expressing human H9 cells and MT-2 target cells assessed as number of syncy... | Bioorg Med Chem Lett 28: 49-52 (2018) Article DOI: 10.1016/j.bmcl.2017.08.049 BindingDB Entry DOI: 10.7270/Q2FX7D2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus 1) | BDBM50452550 (CHEMBL4207660) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Renmin Hospital of Wuhan University Curated by ChEMBL | Assay Description Inhibition of HIV gp41 6-helix bundle formation preincubated with C34 and N36 for 30 mins | Bioorg Med Chem Lett 28: 49-52 (2018) Article DOI: 10.1016/j.bmcl.2017.08.049 BindingDB Entry DOI: 10.7270/Q2FX7D2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus 1) | BDBM50452549 (CHEMBL4203285) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Renmin Hospital of Wuhan University Curated by ChEMBL | Assay Description Inhibition of HIV gp41 6-helix bundle formation preincubated with C34 and N36 for 30 mins | Bioorg Med Chem Lett 28: 49-52 (2018) Article DOI: 10.1016/j.bmcl.2017.08.049 BindingDB Entry DOI: 10.7270/Q2FX7D2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50579949 (CHEMBL5084034) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 154 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50579950 (CHEMBL5080233) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 189 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50579951 (CHEMBL5078349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 202 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50579952 (CHEMBL5076581) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50579953 (CHEMBL5085104) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50579948 (CHEMBL5080666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu-opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01631 BindingDB Entry DOI: 10.7270/Q2988BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||