

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026058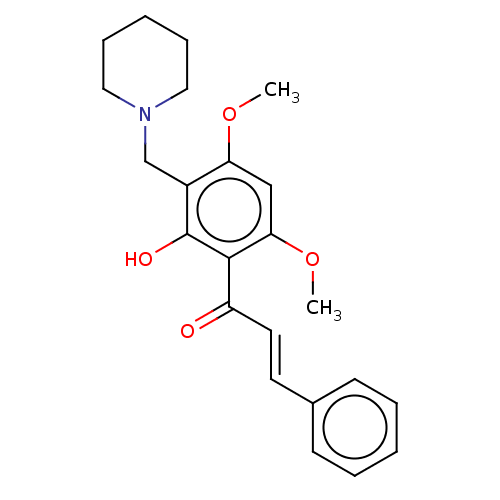 (CHEMBL3335259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE by Michaelis-Menten equation | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50393715 (CHEMBL2158994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Non-competitive inhibition of AChE (unknown origin) incubated for 25 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026058 (CHEMBL3335259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate by Michaelis-Menten equation | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50393715 (CHEMBL2158994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Competitive inhibition of AChE (unknown origin) incubated for 25 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50020713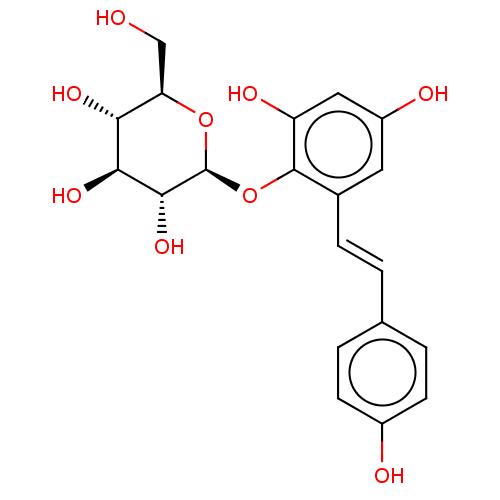 (CHEMBL460860) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 240 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50020713 (CHEMBL460860) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 120 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 120 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50020713 (CHEMBL460860) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 60 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 120 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026058 (CHEMBL3335259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50393715 (CHEMBL2158994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099426 (CHEMBL3339001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099419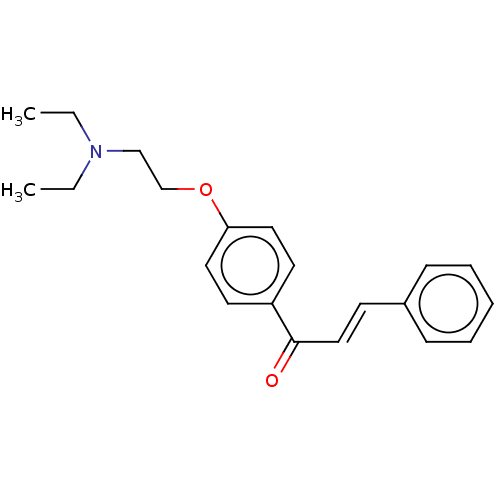 (CHEMBL3337471) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099426 (CHEMBL3339001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099416 (CHEMBL3338992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099425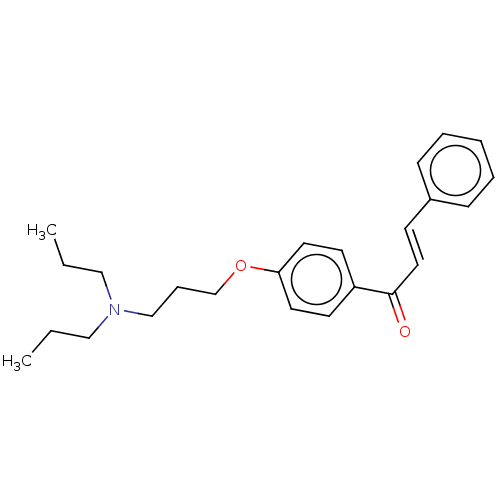 (CHEMBL3339000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099421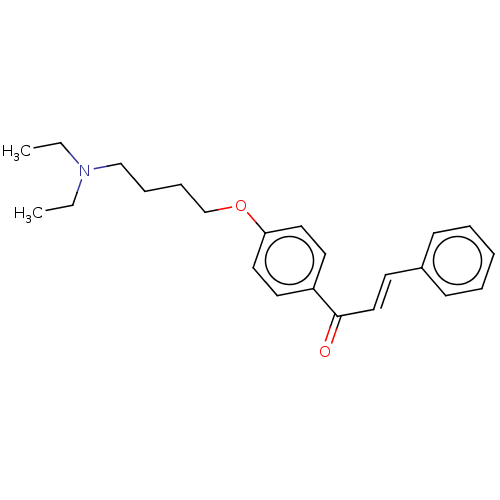 (CHEMBL3338996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099418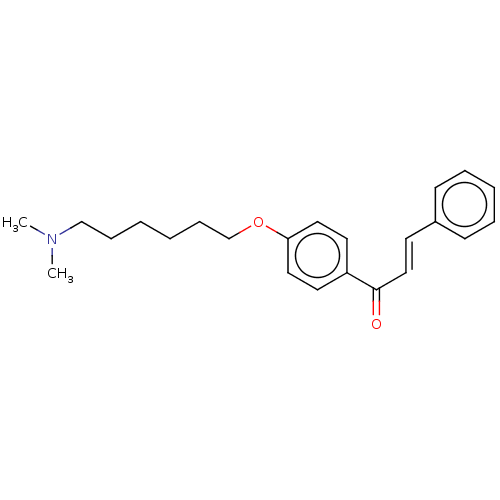 (CHEMBL3338994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099414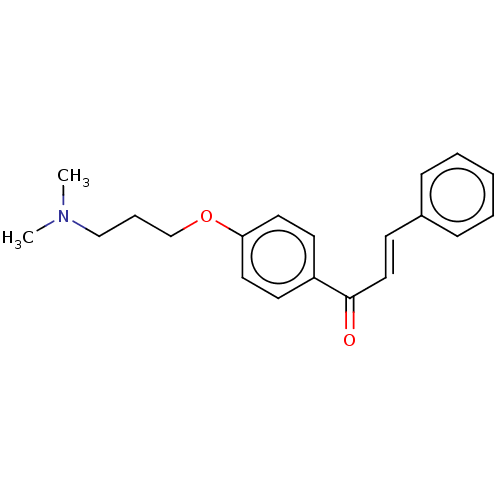 (CHEMBL3338991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099416 (CHEMBL3338992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026061 (CHEMBL3335261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099417 (CHEMBL3338993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099418 (CHEMBL3338994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026062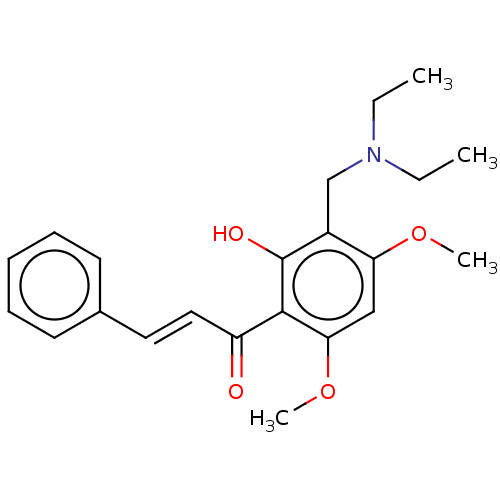 (CHEMBL3335256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026064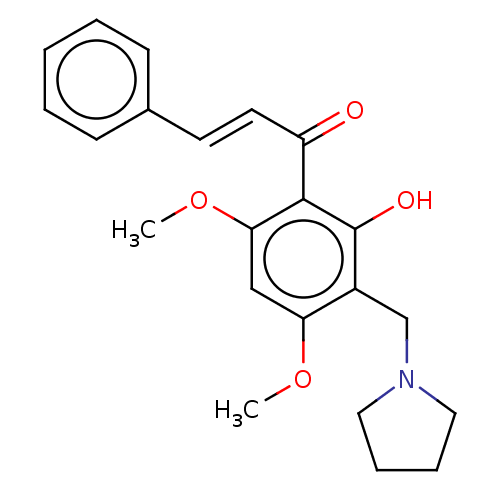 (CHEMBL3335258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099417 (CHEMBL3338993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099423 (CHEMBL3338998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026065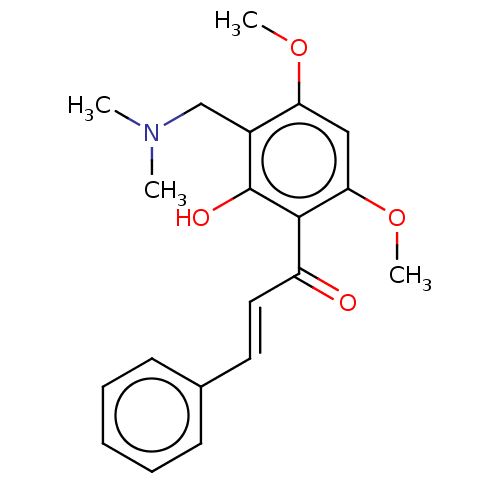 (CHEMBL3335255) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099420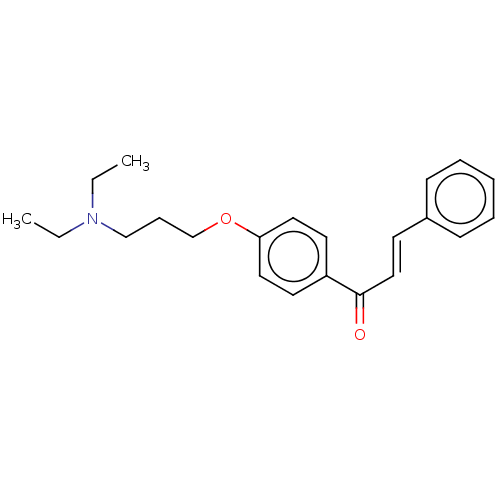 (CHEMBL3338995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50393715 (CHEMBL2158994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099431 (CHEMBL3339006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099420 (CHEMBL3338995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099421 (CHEMBL3338996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099425 (CHEMBL3339000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099422 (CHEMBL3338997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099427 (CHEMBL3339002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099419 (CHEMBL3337471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099414 (CHEMBL3338991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099422 (CHEMBL3338997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099427 (CHEMBL3339002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099428 (CHEMBL3339003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099431 (CHEMBL3339006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099428 (CHEMBL3339003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099423 (CHEMBL3338998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099424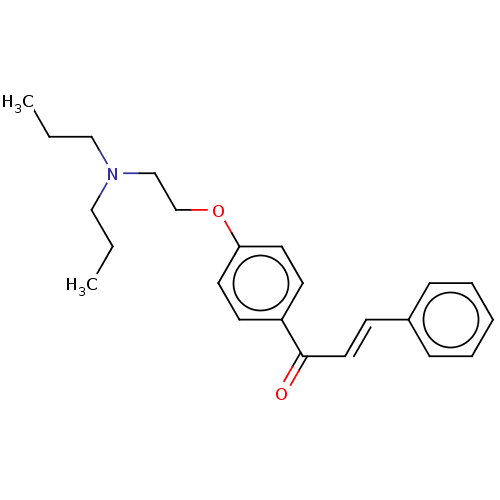 (CHEMBL3338999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of BuChE (unknown origin) using butyrylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50026063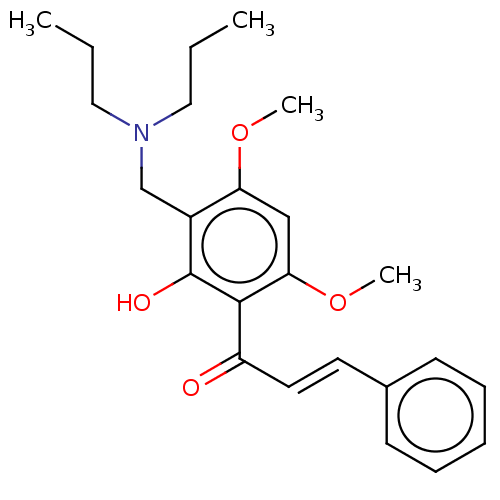 (CHEMBL3335257) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain AChE using acetylthiocholin iodide as substrate preincubated for 25 mins by Ellman's/UV-vis spectroscopy analy... | Bioorg Med Chem Lett 24: 4749-53 (2014) Article DOI: 10.1016/j.bmcl.2014.07.087 BindingDB Entry DOI: 10.7270/Q2PR7XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099424 (CHEMBL3338999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hu'nan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate incubated for 25 mins by Ellman's method | Bioorg Med Chem 22: 6124-33 (2014) Article DOI: 10.1016/j.bmc.2014.08.033 BindingDB Entry DOI: 10.7270/Q2474CNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |