 Found 40 hits with Last Name = 'ichikawa' and Initial = 'y'
Found 40 hits with Last Name = 'ichikawa' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50104412
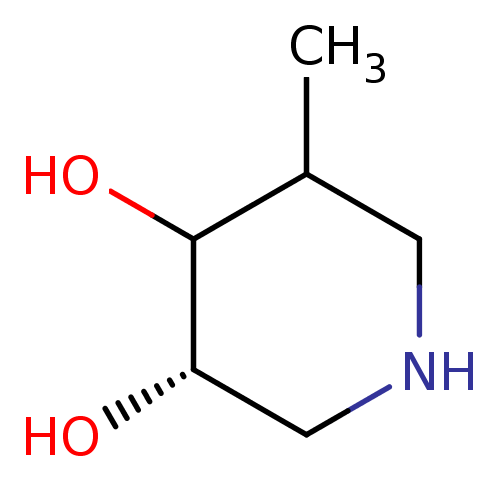
((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884) at pH 6.8 |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50182798
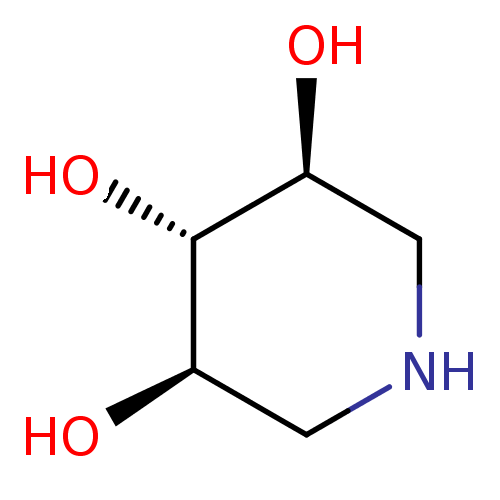
((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50104412

((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50182798

((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50182798

((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-galactosidase from green coffee beans (sigma G 8507). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50288853
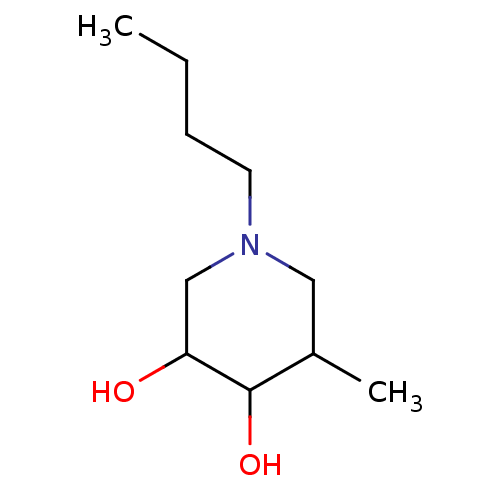
(1-Butyl-5-methyl-piperidine-3,4-diol | CHEMBL35602...)Show InChI InChI=1S/C10H21NO2/c1-3-4-5-11-6-8(2)10(13)9(12)7-11/h8-10,12-13H,3-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50288853

(1-Butyl-5-methyl-piperidine-3,4-diol | CHEMBL35602...)Show InChI InChI=1S/C10H21NO2/c1-3-4-5-11-6-8(2)10(13)9(12)7-11/h8-10,12-13H,3-7H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50182798

((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50104412

((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-galactosidase from green coffee beans (sigma G 8507). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50288853

(1-Butyl-5-methyl-piperidine-3,4-diol | CHEMBL35602...)Show InChI InChI=1S/C10H21NO2/c1-3-4-5-11-6-8(2)10(13)9(12)7-11/h8-10,12-13H,3-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-galactosidase from green coffee beans (sigma G 8507). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50104412

((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50104412

((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50288852
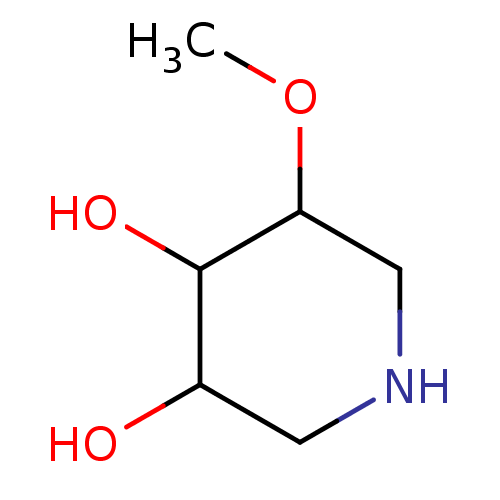
(5-Methoxy-piperidine-3,4-diol | CHEMBL414447)Show InChI InChI=1S/C6H13NO3/c1-10-5-3-7-2-4(8)6(5)9/h4-9H,2-3H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-galactosidase from green coffee beans (sigma G 8507). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50288854

(5-Methylene-piperidine-3,4-diol | CHEMBL150938)Show InChI InChI=1S/C6H11NO2/c1-4-2-7-3-5(8)6(4)9/h5-9H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-galactosidase from green coffee beans (sigma G 8507). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50288854

(5-Methylene-piperidine-3,4-diol | CHEMBL150938)Show InChI InChI=1S/C6H11NO2/c1-4-2-7-3-5(8)6(4)9/h5-9H,1-3H2 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50288852

(5-Methoxy-piperidine-3,4-diol | CHEMBL414447)Show InChI InChI=1S/C6H13NO3/c1-10-5-3-7-2-4(8)6(5)9/h4-9H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50288854

(5-Methylene-piperidine-3,4-diol | CHEMBL150938)Show InChI InChI=1S/C6H11NO2/c1-4-2-7-3-5(8)6(4)9/h5-9H,1-3H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50288852

(5-Methoxy-piperidine-3,4-diol | CHEMBL414447)Show InChI InChI=1S/C6H13NO3/c1-10-5-3-7-2-4(8)6(5)9/h4-9H,2-3H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50288852

(5-Methoxy-piperidine-3,4-diol | CHEMBL414447)Show InChI InChI=1S/C6H13NO3/c1-10-5-3-7-2-4(8)6(5)9/h4-9H,2-3H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50288853

(1-Butyl-5-methyl-piperidine-3,4-diol | CHEMBL35602...)Show InChI InChI=1S/C10H21NO2/c1-3-4-5-11-6-8(2)10(13)9(12)7-11/h8-10,12-13H,3-7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50104412

((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-galactosidase from green coffee beans (sigma G 8507). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50288854

(5-Methylene-piperidine-3,4-diol | CHEMBL150938)Show InChI InChI=1S/C6H11NO2/c1-4-2-7-3-5(8)6(4)9/h5-9H,1-3H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50288854

(5-Methylene-piperidine-3,4-diol | CHEMBL150938)Show InChI InChI=1S/C6H11NO2/c1-4-2-7-3-5(8)6(4)9/h5-9H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50288853

(1-Butyl-5-methyl-piperidine-3,4-diol | CHEMBL35602...)Show InChI InChI=1S/C10H21NO2/c1-3-4-5-11-6-8(2)10(13)9(12)7-11/h8-10,12-13H,3-7H2,1-2H3 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
alpha-1,2-Mannosidase
(Glycine max) | BDBM50288852

(5-Methoxy-piperidine-3,4-diol | CHEMBL414447)Show InChI InChI=1S/C6H13NO3/c1-10-5-3-7-2-4(8)6(5)9/h4-9H,2-3H2,1H3 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of jack bean alpha-mannosidase |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50288854

(5-Methylene-piperidine-3,4-diol | CHEMBL150938)Show InChI InChI=1S/C6H11NO2/c1-4-2-7-3-5(8)6(4)9/h5-9H,1-3H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-glucosidase from yeast(sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50182798

((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50104412

((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-glucosidase from yeast(sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-glucosidase from yeast(sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50288852

(5-Methoxy-piperidine-3,4-diol | CHEMBL414447)Show InChI InChI=1S/C6H13NO3/c1-10-5-3-7-2-4(8)6(5)9/h4-9H,2-3H2,1H3 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-glucosidase from yeast(sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50288853

(1-Butyl-5-methyl-piperidine-3,4-diol | CHEMBL35602...)Show InChI InChI=1S/C10H21NO2/c1-3-4-5-11-6-8(2)10(13)9(12)7-11/h8-10,12-13H,3-7H2,1-2H3 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-glucosidase from yeast(sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50182798

((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-glucosidase from yeast(sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Alpha-(1,3)-fucosyltransferase 10
(Homo sapiens (Human)) | BDBM50421338

(CHEMBL2303754)Show SMILES OC[C@@H]1NC[C@@H](O)[C@@H](O)[C@@H]1O[C@@H]1O[C@@H](CO)[C@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C12H23NO9/c14-2-4-11(7(17)5(16)1-13-4)22-12-10(20)9(19)8(18)6(3-15)21-12/h4-20H,1-3H2/t4-,5+,6-,7+,8-,9-,10-,11+,12-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against fucosyltransferase |
Bioorg Med Chem Lett 1: 425-428 (1991)
Article DOI: 10.1016/S0960-894X(00)80270-X
BindingDB Entry DOI: 10.7270/Q2BK1C7N |
More data for this
Ligand-Target Pair | |
Alpha-(1,3)-fucosyltransferase 10
(Homo sapiens (Human)) | BDBM50421339

(CHEMBL2303753)Show SMILES OC[C@@H]1O[C@@H](O[C@H]2[C@H](O)C=CO[C@H]2CO)[C@@H](O)[C@@H](O)[C@H]1O |r,c:9| Show InChI InChI=1S/C12H20O9/c13-3-6-8(16)9(17)10(18)12(20-6)21-11-5(15)1-2-19-7(11)4-14/h1-2,5-18H,3-4H2/t5-,6+,7+,8+,9+,10+,11+,12+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.25E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against fucosyltransferase |
Bioorg Med Chem Lett 1: 425-428 (1991)
Article DOI: 10.1016/S0960-894X(00)80270-X
BindingDB Entry DOI: 10.7270/Q2BK1C7N |
More data for this
Ligand-Target Pair | |
Alpha-(1,3)-fucosyltransferase 10
(Homo sapiens (Human)) | BDBM50421340

(CHEMBL2303756)Show SMILES CC(=O)N[C@H]1C[C@H](O[C@@H]2O[C@@H](CO)[C@H](O)[C@H](O)[C@@H]2O)[C@H](CO)O[C@@H]1OCC=C |r| Show InChI InChI=1S/C17H29NO10/c1-3-4-25-16-9(18-8(2)21)5-10(11(6-19)27-16)26-17-15(24)14(23)13(22)12(7-20)28-17/h3,9-17,19-20,22-24H,1,4-7H2,2H3,(H,18,21)/t9-,10-,11-,12-,13-,14-,15-,16-,17+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.25E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against fucosyltransferase |
Bioorg Med Chem Lett 1: 425-428 (1991)
Article DOI: 10.1016/S0960-894X(00)80270-X
BindingDB Entry DOI: 10.7270/Q2BK1C7N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data