

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM86674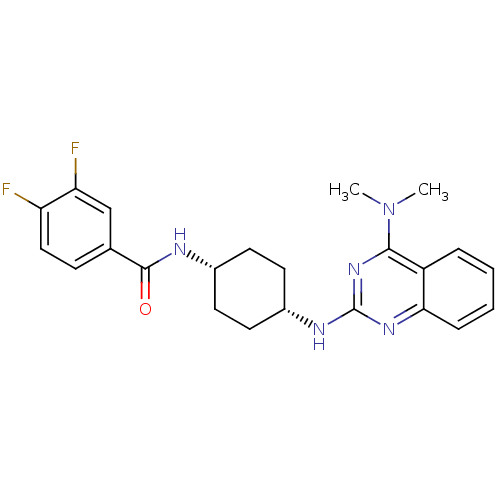 (ATC0175) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 7.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 313: 831-9 (2005) Article DOI: 10.1124/jpet.104.081711 BindingDB Entry DOI: 10.7270/Q2DB80D6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM86674 (ATC0175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 313: 831-9 (2005) Article DOI: 10.1124/jpet.104.081711 BindingDB Entry DOI: 10.7270/Q2DB80D6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM86673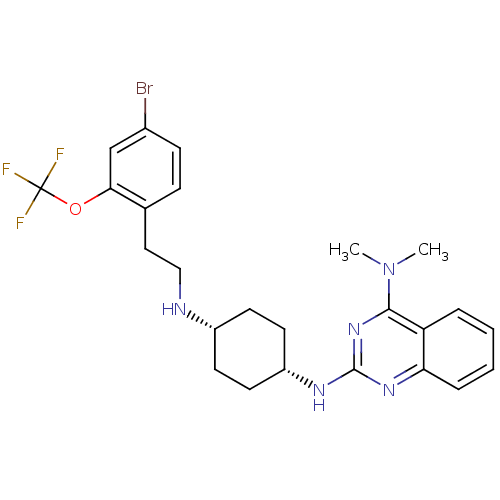 (ATC 0065 | ATC0065 | CAS_510732-84-0) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 313: 831-9 (2005) Article DOI: 10.1124/jpet.104.081711 BindingDB Entry DOI: 10.7270/Q2DB80D6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86674 (ATC0175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 313: 831-9 (2005) Article DOI: 10.1124/jpet.104.081711 BindingDB Entry DOI: 10.7270/Q2DB80D6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM86673 (ATC 0065 | ATC0065 | CAS_510732-84-0) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 313: 831-9 (2005) Article DOI: 10.1124/jpet.104.081711 BindingDB Entry DOI: 10.7270/Q2DB80D6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552997 (CHEMBL4797745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286734 (CHEMBL4172988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491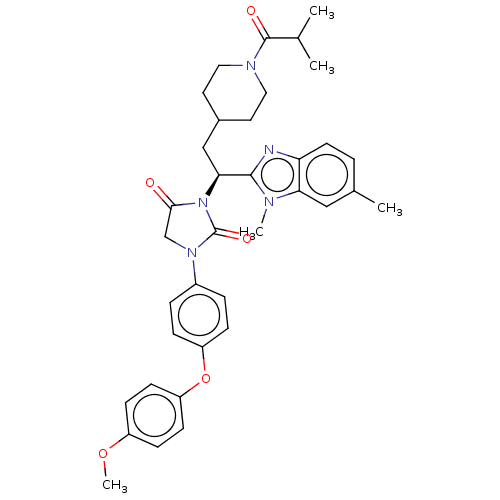 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510487 (CHEMBL4569266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286761 (CHEMBL4169187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286736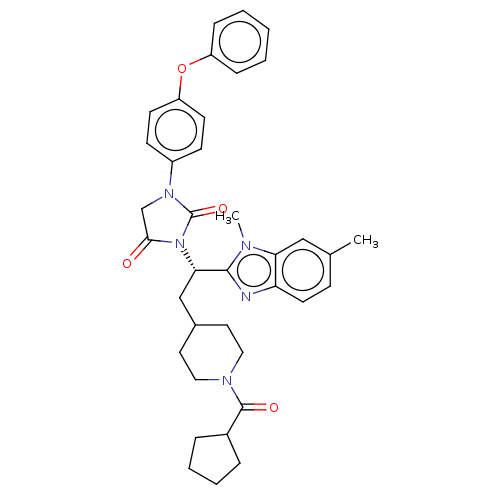 (CHEMBL4161262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50553000 (CHEMBL4783205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286763 (CHEMBL4169596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510495 (CHEMBL4453417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510495 (CHEMBL4453417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552999 (CHEMBL4749439) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286733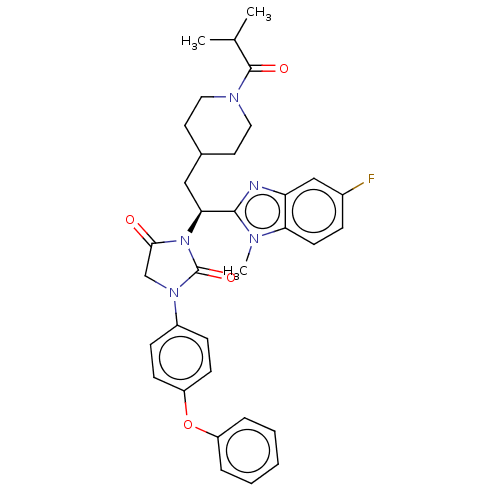 (CHEMBL4162312) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552998 (CHEMBL4783777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552996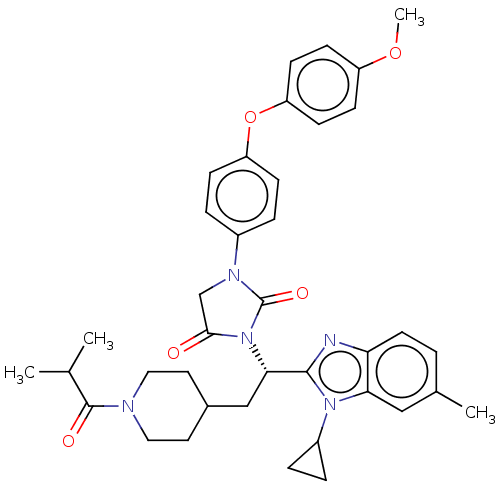 (CHEMBL4785930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286762 (CHEMBL4159402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074866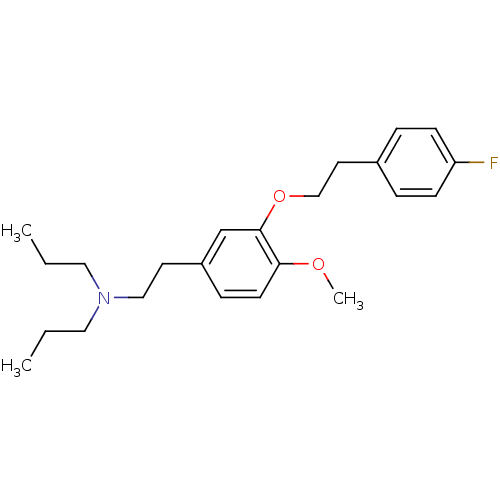 ((2-{3-[2-(4-Fluoro-phenyl)-ethoxy]-4-methoxy-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286764 (CHEMBL4176369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074846 (CHEMBL545597 | [3-(4-Methoxy-3-phenethyloxy-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074867 ((2-{4-Methoxy-3-[2-(4-methoxy-phenyl)-ethoxy]-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50170660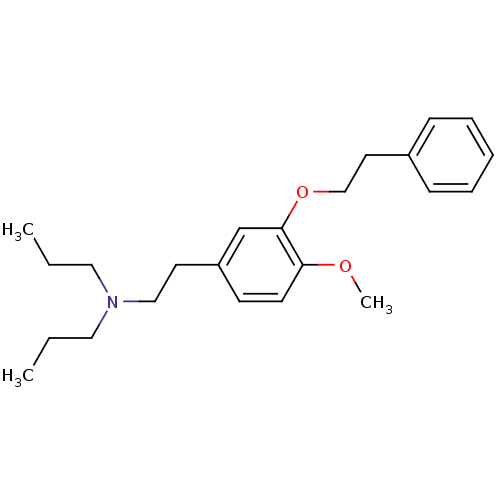 (CHEMBL190883 | CHEMBL521582 | N,N-dipropyl-2-[4-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166561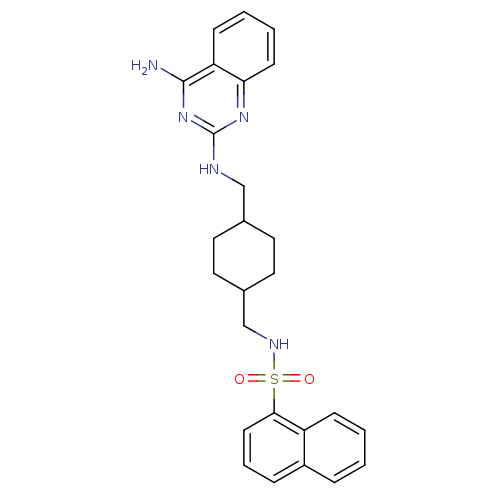 (CHEMBL195380 | Naphthalene-1-sulfonic acid {4-[(4-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074859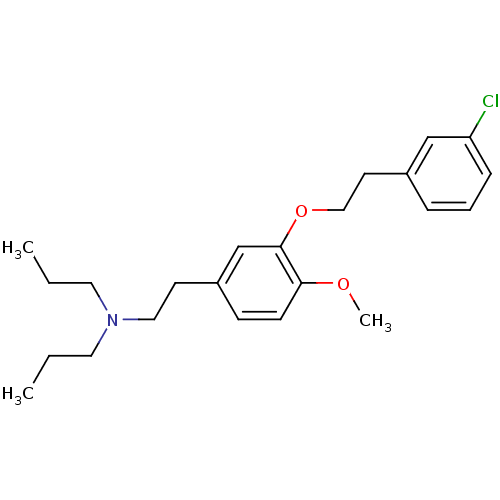 ((2-{3-[2-(3-Chloro-phenyl)-ethoxy]-4-methoxy-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166554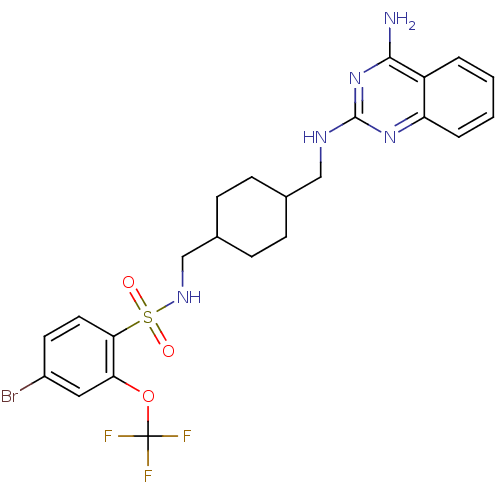 (CHEMBL192266 | N-{4-[(4-Amino-quinazolin-2-ylamino...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074828 (CHEMBL11290 | {2-[3-Methoxy-2-(3-phenyl-propoxy)-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074827 (CHEMBL542298 | Hexyl-[2-(4-methoxy-3-phenethyloxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50553001 (CHEMBL4778665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302559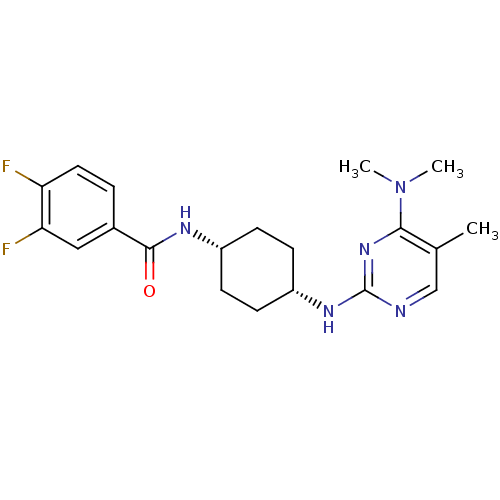 (CHEMBL578049 | N-(cis-4-{[4-(Dimethylamino)-5-meth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50170193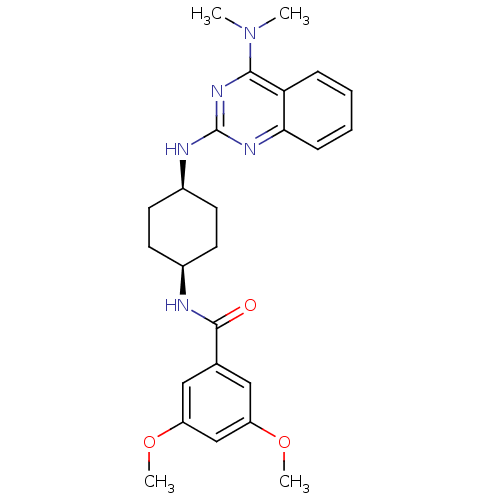 (CHEMBL360081 | N-[4-(4-Dimethylamino-quinazolin-2-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human CA-MCH-R1 (Melanin-concentrating hormone receptor 1) by using [125I](Phe13,Tyr19)MCH as radioligand expressed in HE... | Bioorg Med Chem Lett 15: 3853-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.121 BindingDB Entry DOI: 10.7270/Q23R0TM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302561 (CHEMBL568208 | N-((cis)-4-(4-(dimethylamino)-5,6-d...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50064943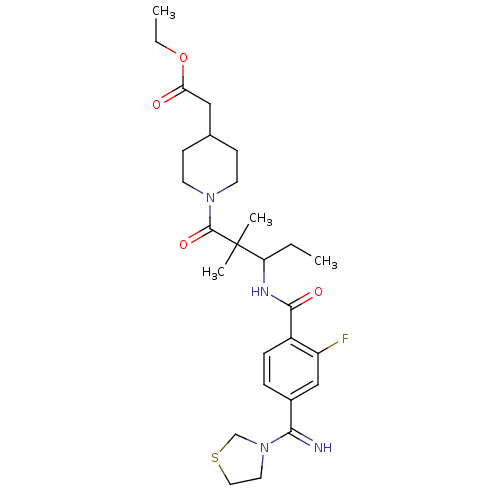 ((1-{3-[2-Fluoro-4-(imino-thiazolidin-3-yl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation Curated by ChEMBL | Assay Description Binding affinity to human fibrinogen receptor | J Med Chem 41: 2345-60 (1998) Article DOI: 10.1021/jm980126v BindingDB Entry DOI: 10.7270/Q2WD3ZPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510488 (CHEMBL4519419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166552 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50302552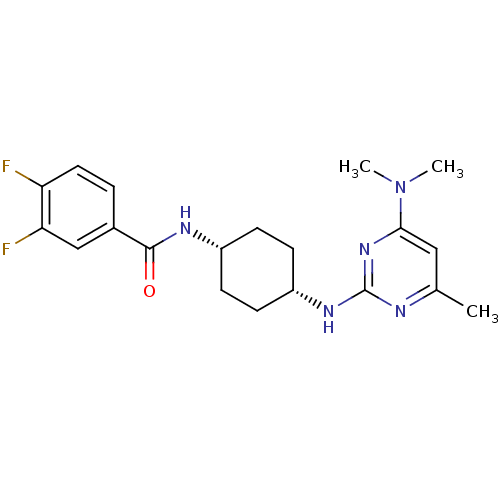 (CHEMBL578166 | N-((cis)-4-(4-(dimethylamino)-6-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CART form of human MCH1 receptor expressed in HEK293 cells coexpressing Galphaq assessed as inhibition of MCH-induced intracel... | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50064930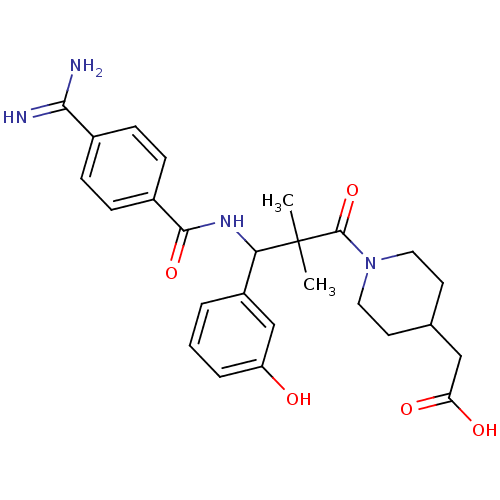 (CHEMBL77113 | {1-[3-(4-Carbamimidoyl-benzoylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation Curated by ChEMBL | Assay Description Binding affinity to human fibrinogen receptor | J Med Chem 41: 2345-60 (1998) Article DOI: 10.1021/jm980126v BindingDB Entry DOI: 10.7270/Q2WD3ZPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50064941 (CHEMBL78295 | {1-[3-(4-Carbamimidoyl-2-fluoro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation Curated by ChEMBL | Assay Description Binding affinity to human fibrinogen receptor | J Med Chem 41: 2345-60 (1998) Article DOI: 10.1021/jm980126v BindingDB Entry DOI: 10.7270/Q2WD3ZPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50064942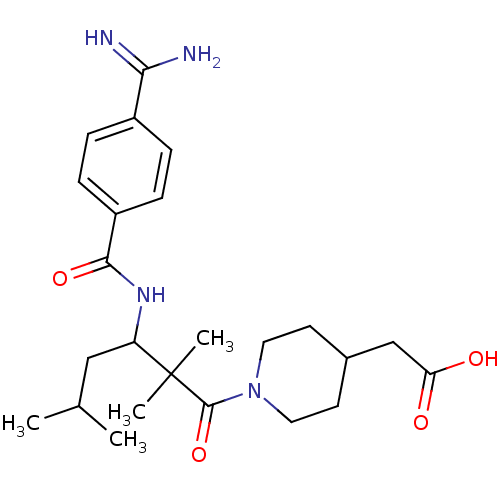 (CHEMBL74908 | {1-[3-(4-Carbamimidoyl-benzoylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation Curated by ChEMBL | Assay Description Binding affinity to human fibrinogen receptor | J Med Chem 41: 2345-60 (1998) Article DOI: 10.1021/jm980126v BindingDB Entry DOI: 10.7270/Q2WD3ZPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50064935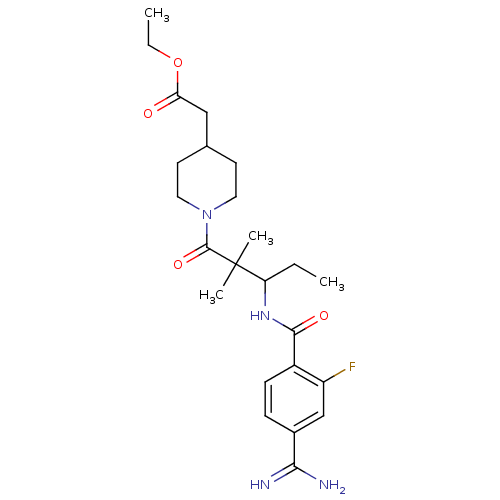 (CHEMBL312368 | {1-[3-(4-Carbamimidoyl-2-fluoro-ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Steel Corporation Curated by ChEMBL | Assay Description Binding affinity to human fibrinogen receptor | J Med Chem 41: 2345-60 (1998) Article DOI: 10.1021/jm980126v BindingDB Entry DOI: 10.7270/Q2WD3ZPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50170189 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against neuropeptide Y receptor type 5 by using [125I]PYY as radioligand expressed in COS-1 cells | Bioorg Med Chem Lett 15: 3853-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.121 BindingDB Entry DOI: 10.7270/Q23R0TM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074839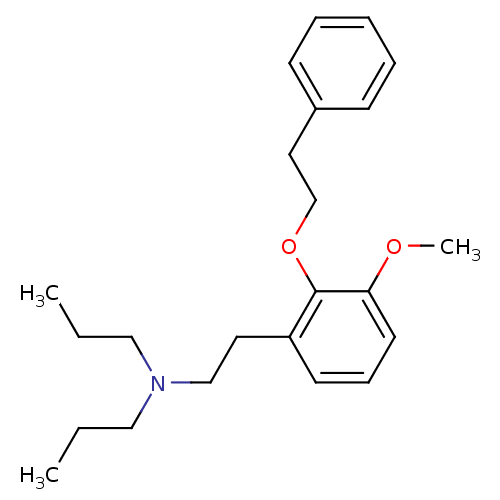 (CHEMBL542047 | [2-(3-Methoxy-2-phenethyloxy-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50402166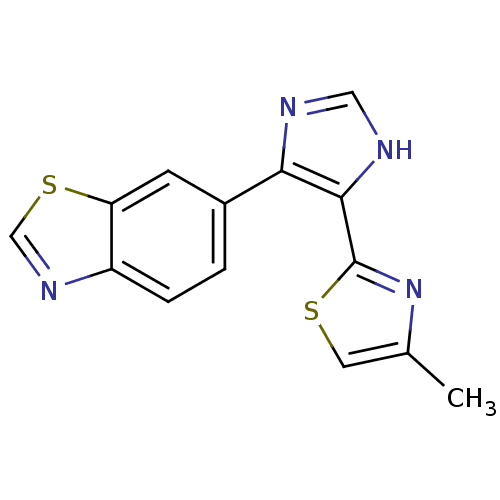 (CHEMBL2207988) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human ALK5 using GST-tagged Smad3 as substrate assessed as substrate phosphorylation after 30 mins by ELISA | Bioorg Med Chem 20: 7128-38 (2012) Article DOI: 10.1016/j.bmc.2012.09.066 BindingDB Entry DOI: 10.7270/Q2542PQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50166555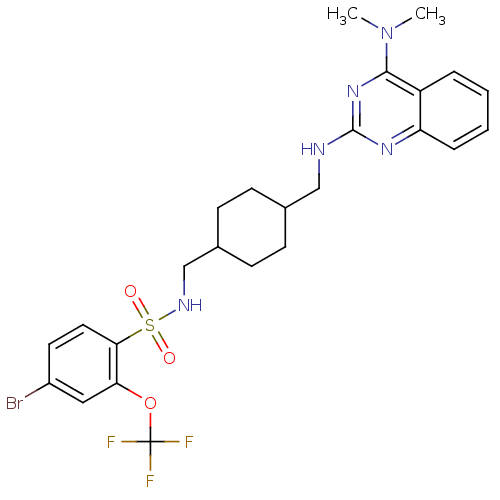 (4-Bromo-N-{4-[(4-dimethylamino-quinazolin-2-ylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibitory activity against constitutively activated human Neuropeptide Y receptor Y5 stransiently expressed in COS-1 cells using [125I]-PYY | Bioorg Med Chem Lett 15: 2565-9 (2005) Article DOI: 10.1016/j.bmcl.2005.03.052 BindingDB Entry DOI: 10.7270/Q2Z89BXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50074865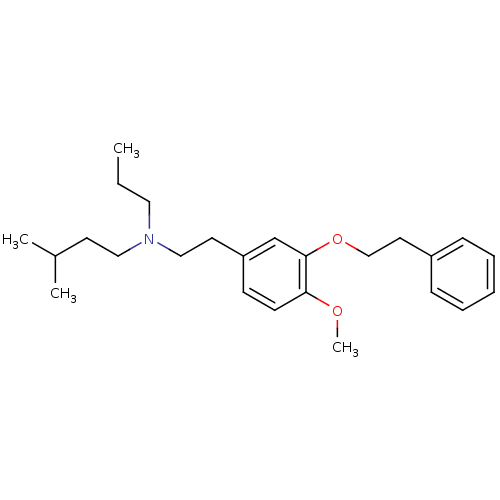 (CHEMBL542295 | [2-(4-Methoxy-3-phenethyloxy-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H]3-PPP binding to Sigma opioid receptor type 1 in rat brain membrane | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1194 total ) | Next | Last >> |