 Found 137 hits with Last Name = 'liu' and Initial = 'yq'
Found 137 hits with Last Name = 'liu' and Initial = 'yq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518668
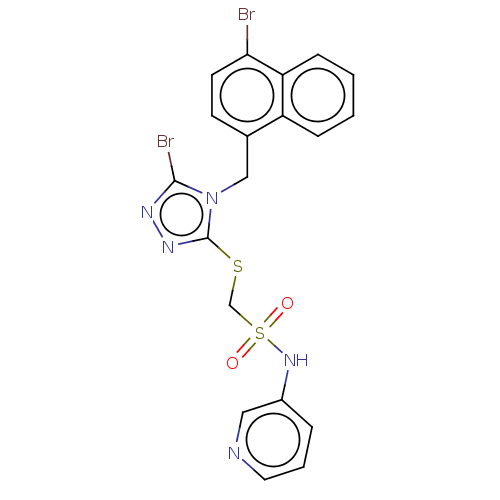
(CHEMBL4546569)Show SMILES Brc1nnc(SCS(=O)(=O)Nc2cccnc2)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C19H15Br2N5O2S2/c20-17-8-7-13(15-5-1-2-6-16(15)17)11-26-18(21)23-24-19(26)29-12-30(27,28)25-14-4-3-9-22-10-14/h1-10,25H,11-12H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518676
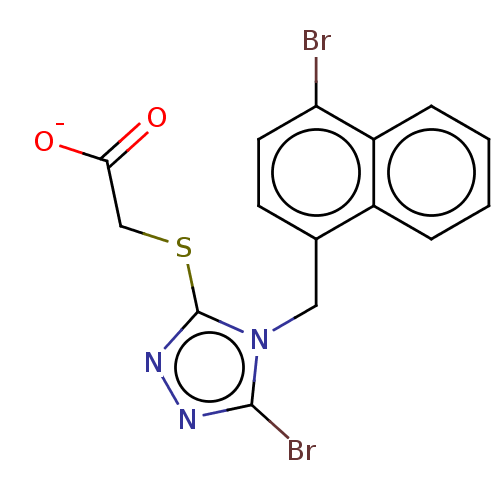
(CHEMBL4453888)Show SMILES [Na;v0+].[#8-]-[#6](=O)-[#6]-[#16]-c1nnc(Br)n1-[#6]-c1ccc(Br)c2ccccc12 Show InChI InChI=1S/C15H11Br2N3O2S/c16-12-6-5-9(10-3-1-2-4-11(10)12)7-20-14(17)18-19-15(20)23-8-13(21)22/h1-6H,7-8H2,(H,21,22)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067478
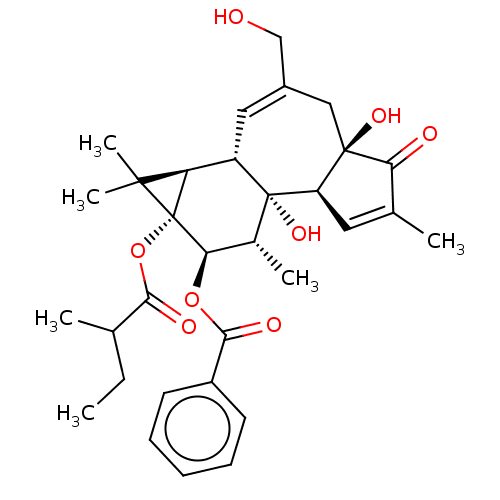
(CHEMBL3400659)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)c3ccccc3)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C32H40O8/c1-7-17(2)27(35)40-32-24(29(32,5)6)22-14-20(16-33)15-30(37)23(13-18(3)25(30)34)31(22,38)19(4)26(32)39-28(36)21-11-9-8-10-12-21/h8-14,17,19,22-24,26,33,37-38H,7,15-16H2,1-6H3/t17?,19-,22+,23-,24-,26-,30-,31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518675
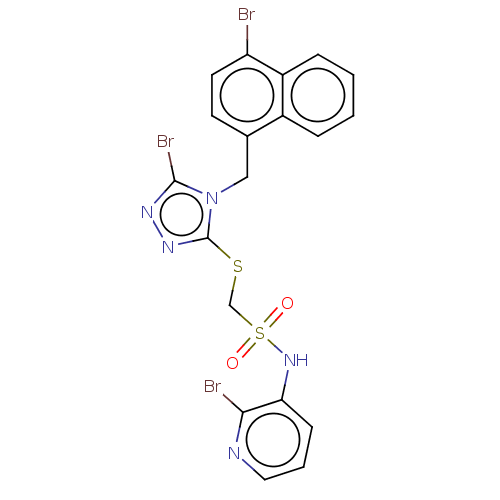
(CHEMBL4458898)Show SMILES Brc1nnc(SCS(=O)(=O)Nc2cccnc2Br)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C19H14Br3N5O2S2/c20-15-8-7-12(13-4-1-2-5-14(13)15)10-27-18(22)24-25-19(27)30-11-31(28,29)26-16-6-3-9-23-17(16)21/h1-9,26H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518679
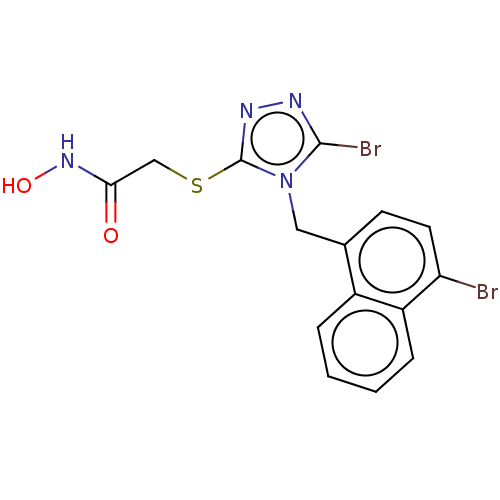
(CHEMBL4468962)Show InChI InChI=1S/C15H12Br2N4O2S/c16-12-6-5-9(10-3-1-2-4-11(10)12)7-21-14(17)18-19-15(21)24-8-13(22)20-23/h1-6,23H,7-8H2,(H,20,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518673
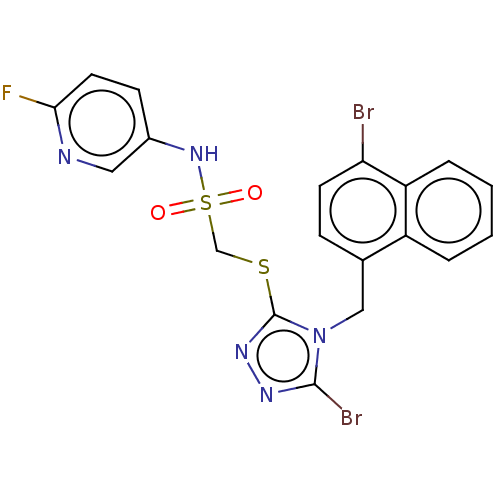
(CHEMBL4448964)Show SMILES Fc1ccc(NS(=O)(=O)CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)cn1 Show InChI InChI=1S/C19H14Br2FN5O2S2/c20-16-7-5-12(14-3-1-2-4-15(14)16)10-27-18(21)24-25-19(27)30-11-31(28,29)26-13-6-8-17(22)23-9-13/h1-9,26H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518665
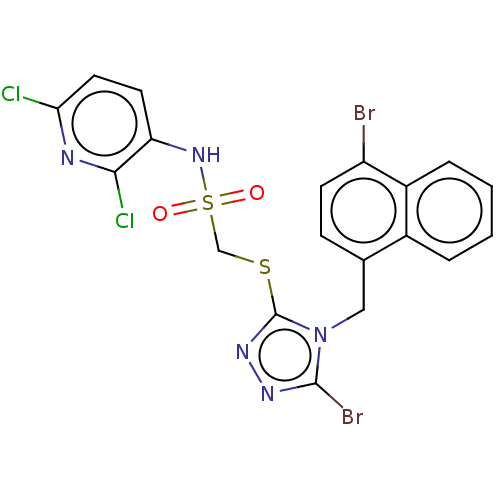
(CHEMBL4445587)Show SMILES Clc1ccc(NS(=O)(=O)CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)c(Cl)n1 Show InChI InChI=1S/C19H13Br2Cl2N5O2S2/c20-14-6-5-11(12-3-1-2-4-13(12)14)9-28-18(21)25-26-19(28)31-10-32(29,30)27-15-7-8-16(22)24-17(15)23/h1-8,27H,9-10H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518667
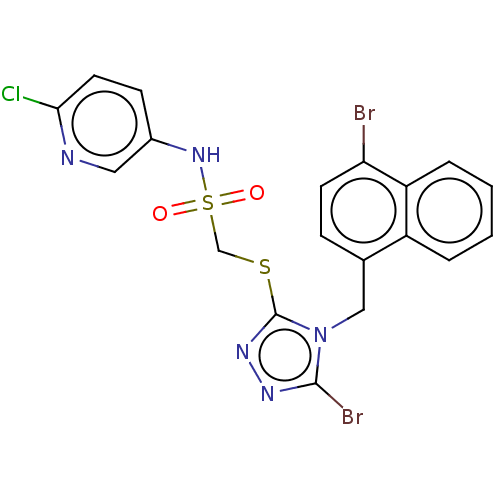
(CHEMBL4585746)Show SMILES Clc1ccc(NS(=O)(=O)CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)cn1 Show InChI InChI=1S/C19H14Br2ClN5O2S2/c20-16-7-5-12(14-3-1-2-4-15(14)16)10-27-18(21)24-25-19(27)30-11-31(28,29)26-13-6-8-17(22)23-9-13/h1-9,26H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518661
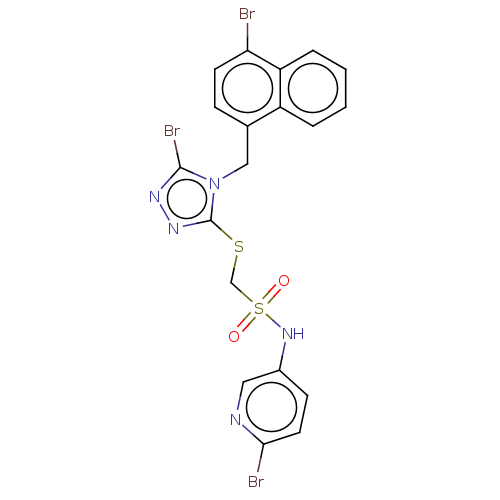
(CHEMBL4554148)Show SMILES Brc1nnc(SCS(=O)(=O)Nc2ccc(Br)nc2)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C19H14Br3N5O2S2/c20-16-7-5-12(14-3-1-2-4-15(14)16)10-27-18(22)24-25-19(27)30-11-31(28,29)26-13-6-8-17(21)23-9-13/h1-9,26H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518666
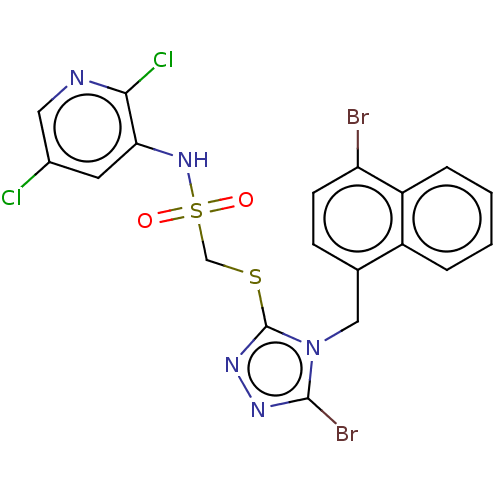
(CHEMBL4556590)Show SMILES Clc1cnc(Cl)c(NS(=O)(=O)CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)c1 Show InChI InChI=1S/C19H13Br2Cl2N5O2S2/c20-15-6-5-11(13-3-1-2-4-14(13)15)9-28-18(21)25-26-19(28)31-10-32(29,30)27-16-7-12(22)8-24-17(16)23/h1-8,27H,9-10H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518671
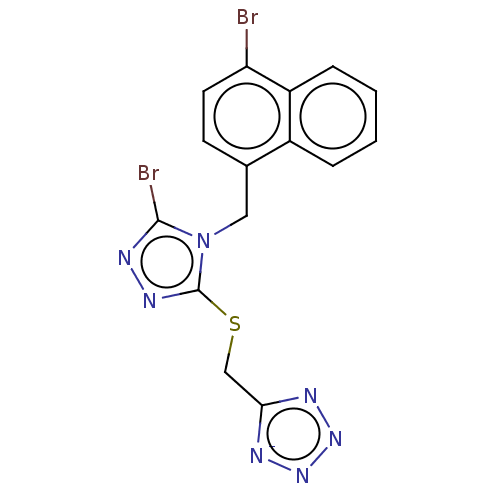
(CHEMBL4560122)Show SMILES [Na;v0+].Brc1nnc(-[#16]-[#6]-c2nnn[n-]2)n1-[#6]-c1ccc(Br)c2ccccc12 Show InChI InChI=1S/C15H10Br2N7S/c16-12-6-5-9(10-3-1-2-4-11(10)12)7-24-14(17)20-21-15(24)25-8-13-18-22-23-19-13/h1-6H,7-8H2/q-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518680
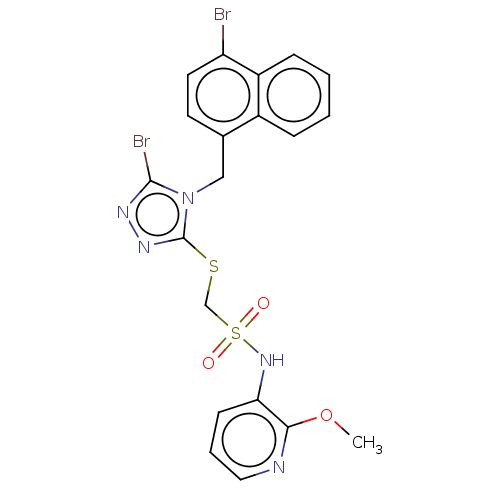
(CHEMBL4573946)Show SMILES COc1ncccc1NS(=O)(=O)CSc1nnc(Br)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C20H17Br2N5O3S2/c1-30-18-17(7-4-10-23-18)26-32(28,29)12-31-20-25-24-19(22)27(20)11-13-8-9-16(21)15-6-3-2-5-14(13)15/h2-10,26H,11-12H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518677
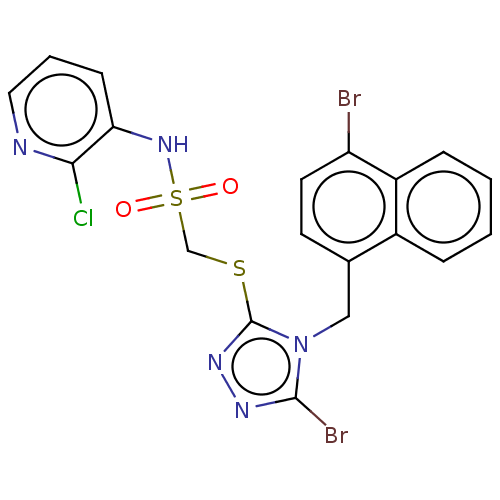
(CHEMBL4467680)Show SMILES Clc1ncccc1NS(=O)(=O)CSc1nnc(Br)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C19H14Br2ClN5O2S2/c20-15-8-7-12(13-4-1-2-5-14(13)15)10-27-18(21)24-25-19(27)30-11-31(28,29)26-16-6-3-9-23-17(16)22/h1-9,26H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518664
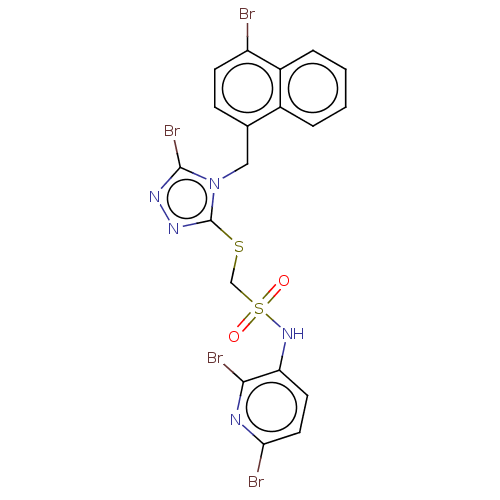
(CHEMBL4473863)Show SMILES Brc1nnc(SCS(=O)(=O)Nc2ccc(Br)nc2Br)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C19H13Br4N5O2S2/c20-14-6-5-11(12-3-1-2-4-13(12)14)9-28-18(23)25-26-19(28)31-10-32(29,30)27-15-7-8-16(21)24-17(15)22/h1-8,27H,9-10H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518678
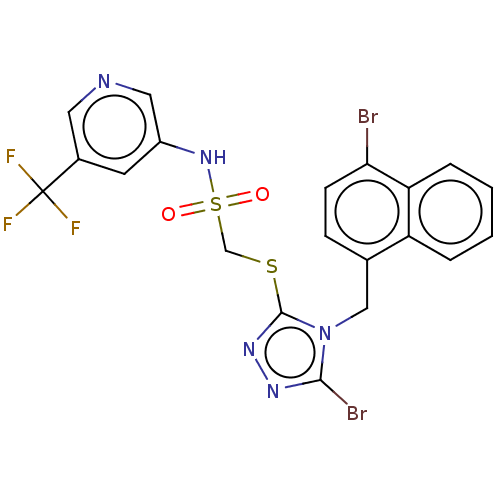
(CHEMBL4516802)Show SMILES FC(F)(F)c1cncc(NS(=O)(=O)CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)c1 Show InChI InChI=1S/C20H14Br2F3N5O2S2/c21-17-6-5-12(15-3-1-2-4-16(15)17)10-30-18(22)27-28-19(30)33-11-34(31,32)29-14-7-13(8-26-9-14)20(23,24)25/h1-9,29H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM37953
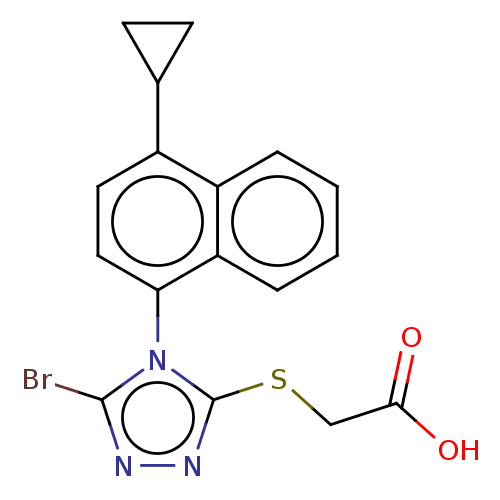
(US10093631, Compound Lesinurad | US10336710, Lesin...)Show SMILES OC(=O)CSc1nnc(Br)n1-c1ccc(C2CC2)c2ccccc12 |(-2.97,1.02,;-1.83,-.01,;-2.15,-1.52,;-.36,.46,;-.04,1.97,;1.42,2.45,;1.9,3.91,;3.44,3.91,;3.91,2.45,;5.38,1.97,;2.67,1.54,;2.67,,;1.33,-.77,;1.33,-2.31,;2.67,-3.08,;2.67,-4.62,;3.44,-5.95,;1.9,-5.95,;4,-2.31,;5.33,-3.08,;6.67,-2.31,;6.67,-.77,;5.33,,;4,-.77,)| Show InChI InChI=1S/C25H32N4O3/c1-18-13-14-19(2)21(16-18)29(17-24(31)27-20-8-3-4-9-20)25(32)12-7-11-23(30)28-22-10-5-6-15-26-22/h5-6,10,13-16,20H,3-4,7-9,11-12,17H2,1-2H3,(H,27,31)(H,26,28,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM37953

(US10093631, Compound Lesinurad | US10336710, Lesin...)Show SMILES OC(=O)CSc1nnc(Br)n1-c1ccc(C2CC2)c2ccccc12 |(-2.97,1.02,;-1.83,-.01,;-2.15,-1.52,;-.36,.46,;-.04,1.97,;1.42,2.45,;1.9,3.91,;3.44,3.91,;3.91,2.45,;5.38,1.97,;2.67,1.54,;2.67,,;1.33,-.77,;1.33,-2.31,;2.67,-3.08,;2.67,-4.62,;3.44,-5.95,;1.9,-5.95,;4,-2.31,;5.33,-3.08,;6.67,-2.31,;6.67,-.77,;5.33,,;4,-.77,)| Show InChI InChI=1S/C25H32N4O3/c1-18-13-14-19(2)21(16-18)29(17-24(31)27-20-8-3-4-9-20)25(32)12-7-11-23(30)28-22-10-5-6-15-26-22/h5-6,10,13-16,20H,3-4,7-9,11-12,17H2,1-2H3,(H,27,31)(H,26,28,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067478

(CHEMBL3400659)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)c3ccccc3)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C32H40O8/c1-7-17(2)27(35)40-32-24(29(32,5)6)22-14-20(16-33)15-30(37)23(13-18(3)25(30)34)31(22,38)19(4)26(32)39-28(36)21-11-9-8-10-12-21/h8-14,17,19,22-24,26,33,37-38H,7,15-16H2,1-6H3/t17?,19-,22+,23-,24-,26-,30-,31-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518662
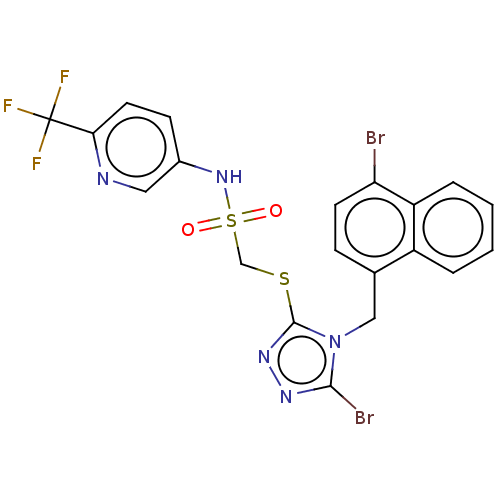
(CHEMBL4436938)Show SMILES FC(F)(F)c1ccc(NS(=O)(=O)CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)cn1 Show InChI InChI=1S/C20H14Br2F3N5O2S2/c21-16-7-5-12(14-3-1-2-4-15(14)16)10-30-18(22)27-28-19(30)33-11-34(31,32)29-13-6-8-17(26-9-13)20(23,24)25/h1-9,29H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518663
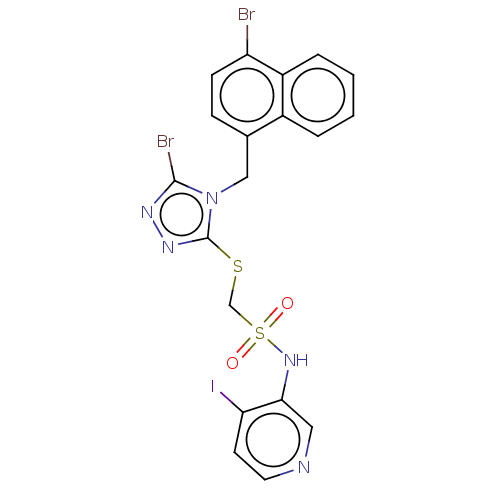
(CHEMBL4551589)Show SMILES Brc1nnc(SCS(=O)(=O)Nc2cnccc2I)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C19H14Br2IN5O2S2/c20-15-6-5-12(13-3-1-2-4-14(13)15)10-27-18(21)24-25-19(27)30-11-31(28,29)26-17-9-23-8-7-16(17)22/h1-9,26H,10-11H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518670
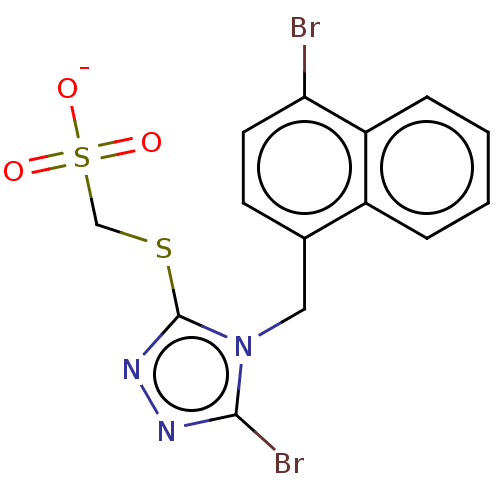
(CHEMBL4568207)Show SMILES [Na;v0+].[#8-]S(=O)(=O)[#6]-[#16]-c1nnc(Br)n1-[#6]-c1ccc(Br)c2ccccc12 Show InChI InChI=1S/C14H11Br2N3O3S2/c15-12-6-5-9(10-3-1-2-4-11(10)12)7-19-13(16)17-18-14(19)23-8-24(20,21)22/h1-6H,7-8H2,(H,20,21,22)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518669
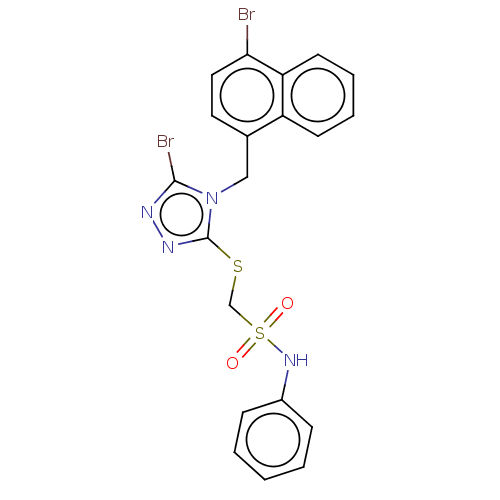
(CHEMBL4517057)Show SMILES Brc1nnc(SCS(=O)(=O)Nc2ccccc2)n1Cc1ccc(Br)c2ccccc12 Show InChI InChI=1S/C20H16Br2N4O2S2/c21-18-11-10-14(16-8-4-5-9-17(16)18)12-26-19(22)23-24-20(26)29-13-30(27,28)25-15-6-2-1-3-7-15/h1-11,25H,12-13H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067483
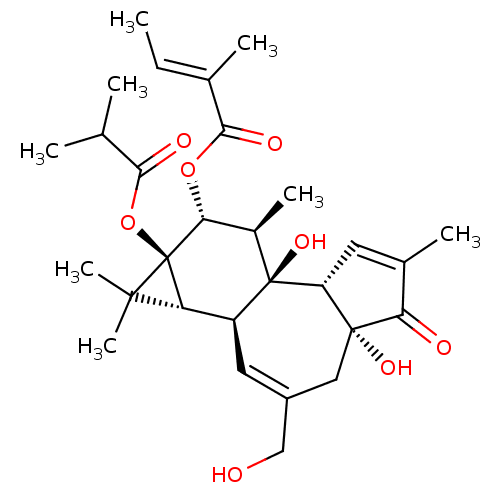
(CHEMBL2375787)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)C(\C)=C\C)[C@@]1(OC(=O)C(C)C)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C29H40O8/c1-9-15(4)25(33)36-23-17(6)28(35)19(21-26(7,8)29(21,23)37-24(32)14(2)3)11-18(13-30)12-27(34)20(28)10-16(5)22(27)31/h9-11,14,17,19-21,23,30,34-35H,12-13H2,1-8H3/b15-9+/t17-,19+,20-,21-,23-,27-,28-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067490
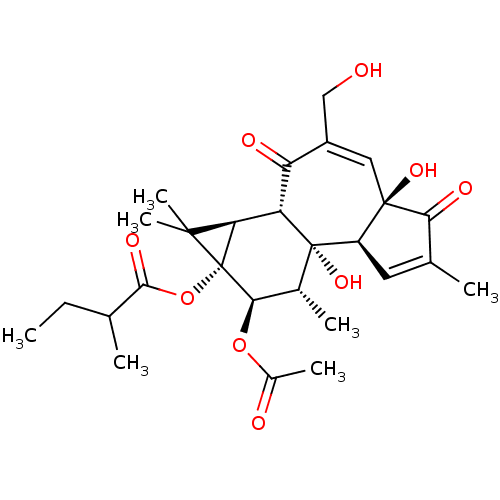
(CHEMBL3400662)Show SMILES [H][C@]12[C@]3([H])C(=O)C(CO)=C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(C)=O)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:8,15| Show InChI InChI=1S/C27H36O9/c1-8-12(2)23(32)36-27-20(24(27,6)7)18-19(30)16(11-28)10-25(33)17(9-13(3)21(25)31)26(18,34)14(4)22(27)35-15(5)29/h9-10,12,14,17-18,20,22,28,33-34H,8,11H2,1-7H3/t12?,14-,17-,18+,20?,22-,25-,26+,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067490

(CHEMBL3400662)Show SMILES [H][C@]12[C@]3([H])C(=O)C(CO)=C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(C)=O)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:8,15| Show InChI InChI=1S/C27H36O9/c1-8-12(2)23(32)36-27-20(24(27,6)7)18-19(30)16(11-28)10-25(33)17(9-13(3)21(25)31)26(18,34)14(4)22(27)35-15(5)29/h9-10,12,14,17-18,20,22,28,33-34H,8,11H2,1-7H3/t12?,14-,17-,18+,20?,22-,25-,26+,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067486
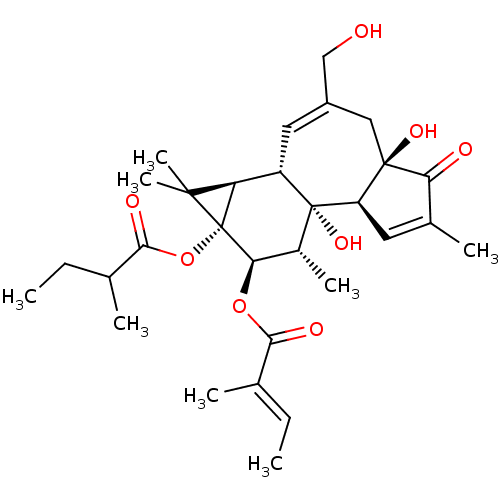
(CHEMBL2375786)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)C(\C)=C\C)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C30H42O8/c1-9-15(3)25(33)37-24-18(6)29(36)20(22-27(7,8)30(22,24)38-26(34)16(4)10-2)12-19(14-31)13-28(35)21(29)11-17(5)23(28)32/h9,11-12,16,18,20-22,24,31,35-36H,10,13-14H2,1-8H3/b15-9+/t16?,18-,20+,21-,22-,24-,28-,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067481

(CHEMBL3400661)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@@]4([H])C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](O)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C25H36O6/c1-7-12(2)22(29)31-25-20(23(25,5)6)18-10-15(11-26)9-16-17(8-13(3)19(16)27)24(18,30)14(4)21(25)28/h8,10,12,14,16-18,20-21,26,28,30H,7,9,11H2,1-6H3/t12?,14-,16-,17-,18+,20-,21-,24+,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067481

(CHEMBL3400661)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@@]4([H])C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](O)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C25H36O6/c1-7-12(2)22(29)31-25-20(23(25,5)6)18-10-15(11-26)9-16-17(8-13(3)19(16)27)24(18,30)14(4)21(25)28/h8,10,12,14,16-18,20-21,26,28,30H,7,9,11H2,1-6H3/t12?,14-,16-,17-,18+,20-,21-,24+,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067477
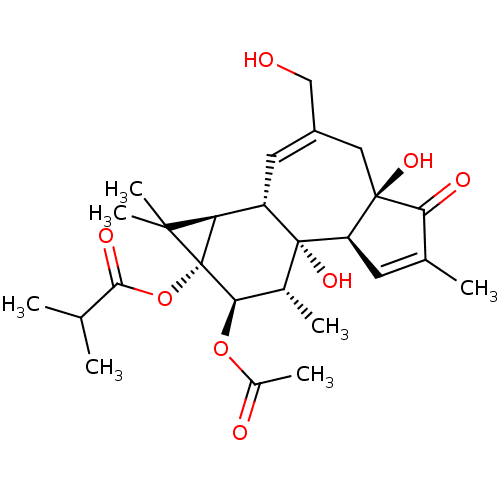
(CHEMBL3400658)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(C)=O)[C@@]1(OC(=O)C(C)C)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C26H36O8/c1-12(2)22(30)34-26-19(23(26,6)7)17-9-16(11-27)10-24(31)18(8-13(3)20(24)29)25(17,32)14(4)21(26)33-15(5)28/h8-9,12,14,17-19,21,27,31-32H,10-11H2,1-7H3/t14-,17+,18-,19-,21-,24-,25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067477

(CHEMBL3400658)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(C)=O)[C@@]1(OC(=O)C(C)C)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C26H36O8/c1-12(2)22(30)34-26-19(23(26,6)7)17-9-16(11-27)10-24(31)18(8-13(3)20(24)29)25(17,32)14(4)21(26)33-15(5)28/h8-9,12,14,17-19,21,27,31-32H,10-11H2,1-7H3/t14-,17+,18-,19-,21-,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067480
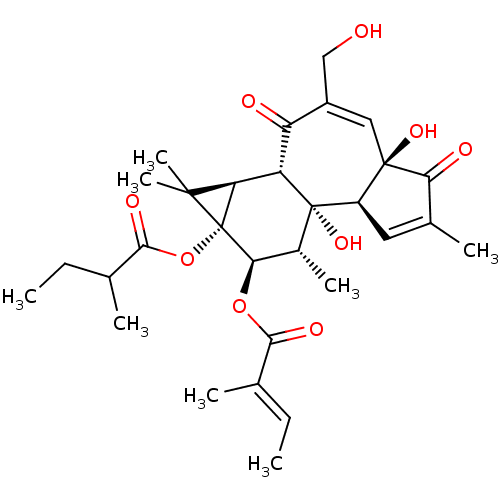
(CHEMBL3400660)Show SMILES [H][C@]12[C@]3([H])C(=O)C(CO)=C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)C(\C)=C\C)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:8,15| Show InChI InChI=1S/C30H40O9/c1-9-14(3)25(34)38-24-17(6)29(37)19-11-16(5)23(33)28(19,36)12-18(13-31)21(32)20(29)22-27(7,8)30(22,24)39-26(35)15(4)10-2/h9,11-12,15,17,19-20,22,24,31,36-37H,10,13H2,1-8H3/b14-9+/t15?,17-,19-,20+,22-,24-,28-,29+,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067480

(CHEMBL3400660)Show SMILES [H][C@]12[C@]3([H])C(=O)C(CO)=C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)C(\C)=C\C)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:8,15| Show InChI InChI=1S/C30H40O9/c1-9-14(3)25(34)38-24-17(6)29(37)19-11-16(5)23(33)28(19,36)12-18(13-31)21(32)20(29)22-27(7,8)30(22,24)39-26(35)15(4)10-2/h9,11-12,15,17,19-20,22,24,31,36-37H,10,13H2,1-8H3/b14-9+/t15?,17-,19-,20+,22-,24-,28-,29+,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067483

(CHEMBL2375787)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)C(\C)=C\C)[C@@]1(OC(=O)C(C)C)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C29H40O8/c1-9-15(4)25(33)36-23-17(6)28(35)19(21-26(7,8)29(21,23)37-24(32)14(2)3)11-18(13-30)12-27(34)20(28)10-16(5)22(27)31/h9-11,14,17,19-21,23,30,34-35H,12-13H2,1-8H3/b15-9+/t17-,19+,20-,21-,23-,27-,28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50067486

(CHEMBL2375786)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)[C@@H](OC(=O)C(\C)=C\C)[C@@]1(OC(=O)C(C)CC)C2(C)C |r,c:14,t:4| Show InChI InChI=1S/C30H42O8/c1-9-15(3)25(33)37-24-18(6)29(36)20(22-27(7,8)30(22,24)38-26(34)16(4)10-2)12-19(14-31)13-28(35)21(29)11-17(5)23(28)32/h9,11-12,16,18,20-22,24,31,35-36H,10,13-14H2,1-8H3/b15-9+/t16?,18-,20+,21-,22-,24-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using fluorometric substrate after 15 mins |
Bioorg Med Chem Lett 25: 1986-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.017
BindingDB Entry DOI: 10.7270/Q27946CT |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518672
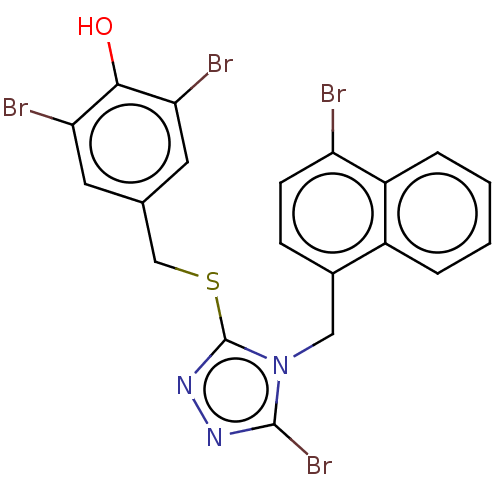
(CHEMBL4594032)Show SMILES Oc1c(Br)cc(CSc2nnc(Br)n2Cc2ccc(Br)c3ccccc23)cc1Br Show InChI InChI=1S/C20H13Br4N3OS/c21-15-6-5-12(13-3-1-2-4-14(13)15)9-27-19(24)25-26-20(27)29-10-11-7-16(22)18(28)17(23)8-11/h1-8,28H,9-10H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260319
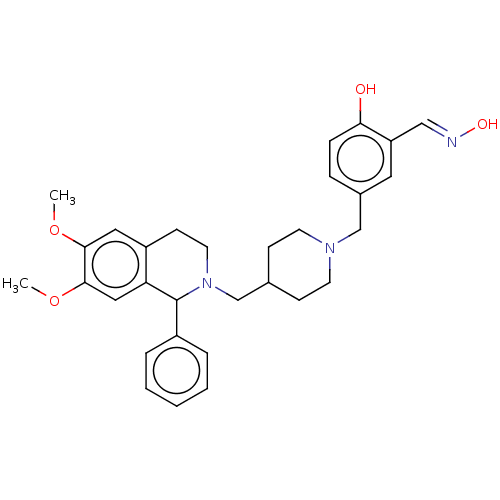
(CHEMBL4104534)Show SMILES COc1cc2CCN(CC3CCN(Cc4ccc(O)c(\C=N\O)c4)CC3)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C31H37N3O4/c1-37-29-17-25-12-15-34(31(24-6-4-3-5-7-24)27(25)18-30(29)38-2)21-22-10-13-33(14-11-22)20-23-8-9-28(35)26(16-23)19-32-36/h3-9,16-19,22,31,35-36H,10-15,20-21H2,1-2H3/b32-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50518674
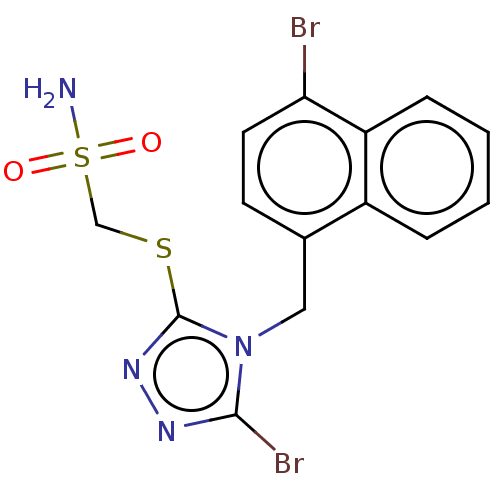
(CHEMBL4459618)Show InChI InChI=1S/C14H12Br2N4O2S2/c15-12-6-5-9(10-3-1-2-4-11(10)12)7-20-13(16)18-19-14(20)23-8-24(17,21)22/h1-6H,7-8H2,(H2,17,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293 cells assessed as reduction in [8-14C]uric acid uptake |
Bioorg Med Chem Lett 29: 383-388 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.036
BindingDB Entry DOI: 10.7270/Q2MP56PF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260318
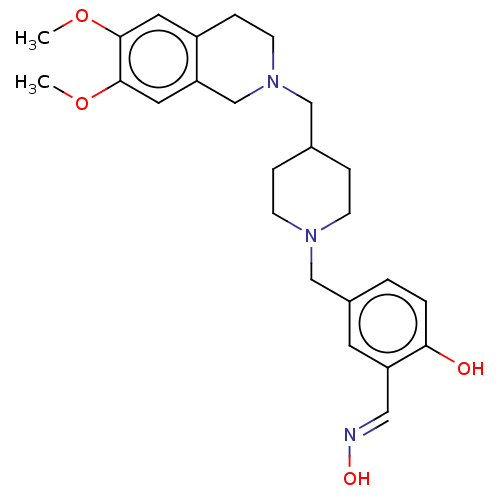
(CHEMBL4061404)Show SMILES COc1cc2CCN(CC3CCN(Cc4ccc(O)c(\C=N\O)c4)CC3)Cc2cc1OC Show InChI InChI=1S/C25H33N3O4/c1-31-24-12-20-7-10-28(17-22(20)13-25(24)32-2)15-18-5-8-27(9-6-18)16-19-3-4-23(29)21(11-19)14-26-30/h3-4,11-14,18,29-30H,5-10,15-17H2,1-2H3/b26-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260307
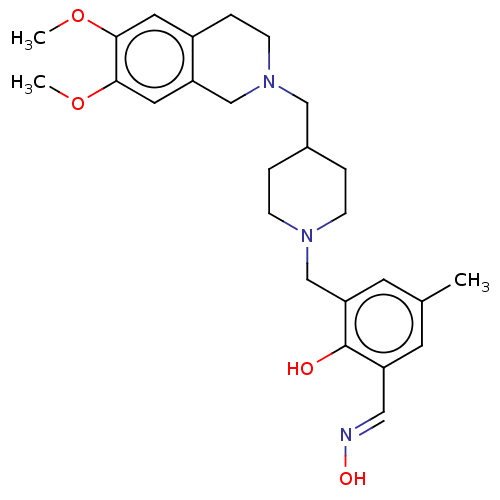
(CHEMBL4089550)Show SMILES COc1cc2CCN(CC3CCN(Cc4cc(C)cc(\C=N\O)c4O)CC3)Cc2cc1OC Show InChI InChI=1S/C26H35N3O4/c1-18-10-21(14-27-31)26(30)23(11-18)17-28-7-4-19(5-8-28)15-29-9-6-20-12-24(32-2)25(33-3)13-22(20)16-29/h10-14,19,30-31H,4-9,15-17H2,1-3H3/b27-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260320
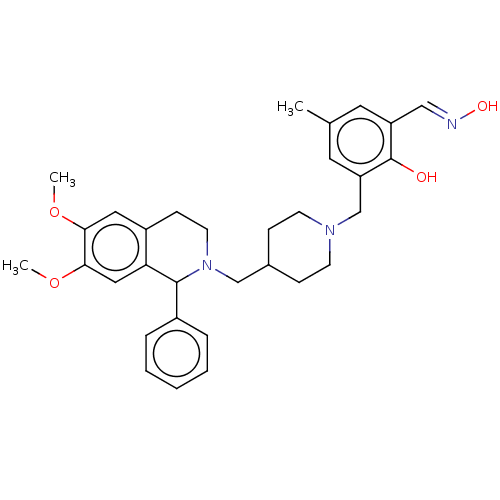
(CHEMBL4094380)Show SMILES COc1cc2CCN(CC3CCN(Cc4cc(C)cc(\C=N\O)c4O)CC3)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C32H39N3O4/c1-22-15-26(19-33-37)32(36)27(16-22)21-34-12-9-23(10-13-34)20-35-14-11-25-17-29(38-2)30(39-3)18-28(25)31(35)24-7-5-4-6-8-24/h4-8,15-19,23,31,36-37H,9-14,20-21H2,1-3H3/b33-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260302
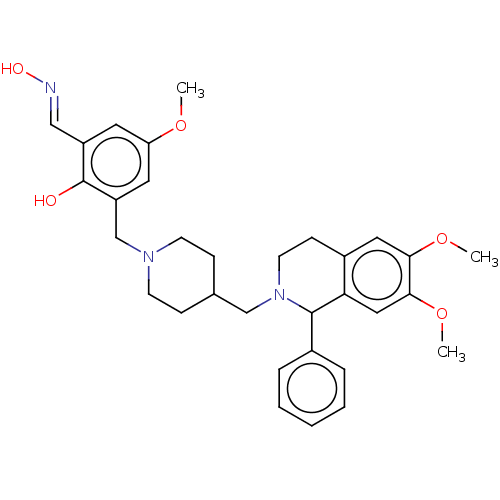
(CHEMBL4064304)Show SMILES COc1cc(CN2CCC(CN3CCc4cc(OC)c(OC)cc4C3c3ccccc3)CC2)c(O)c(\C=N\O)c1 Show InChI InChI=1S/C32H39N3O5/c1-38-27-15-25(19-33-37)32(36)26(16-27)21-34-12-9-22(10-13-34)20-35-14-11-24-17-29(39-2)30(40-3)18-28(24)31(35)23-7-5-4-6-8-23/h4-8,15-19,22,31,36-37H,9-14,20-21H2,1-3H3/b33-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260299
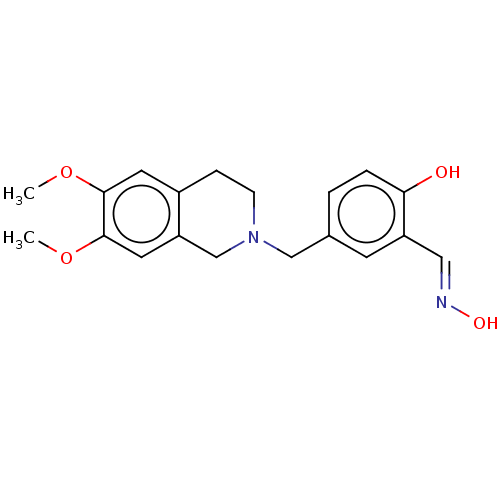
(CHEMBL4088294)Show InChI InChI=1S/C19H22N2O4/c1-24-18-8-14-5-6-21(12-16(14)9-19(18)25-2)11-13-3-4-17(22)15(7-13)10-20-23/h3-4,7-10,22-23H,5-6,11-12H2,1-2H3/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260309
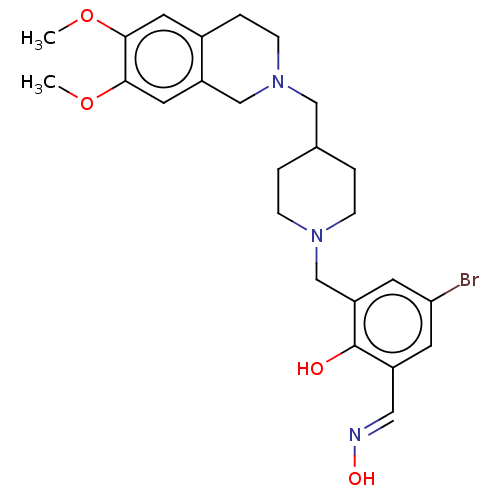
(CHEMBL4077866)Show SMILES COc1cc2CCN(CC3CCN(Cc4cc(Br)cc(\C=N\O)c4O)CC3)Cc2cc1OC Show InChI InChI=1S/C25H32BrN3O4/c1-32-23-11-18-5-8-29(15-20(18)12-24(23)33-2)14-17-3-6-28(7-4-17)16-21-10-22(26)9-19(13-27-31)25(21)30/h9-13,17,30-31H,3-8,14-16H2,1-2H3/b27-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260313
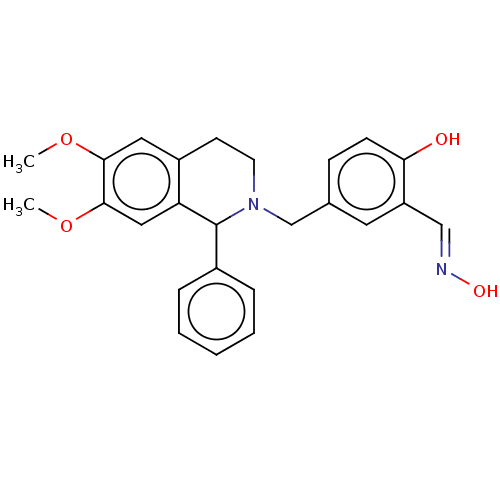
(CHEMBL4100639)Show SMILES COc1cc2CCN(Cc3ccc(O)c(\C=N\O)c3)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C25H26N2O4/c1-30-23-13-19-10-11-27(16-17-8-9-22(28)20(12-17)15-26-29)25(18-6-4-3-5-7-18)21(19)14-24(23)31-2/h3-9,12-15,25,28-29H,10-11,16H2,1-2H3/b26-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260308
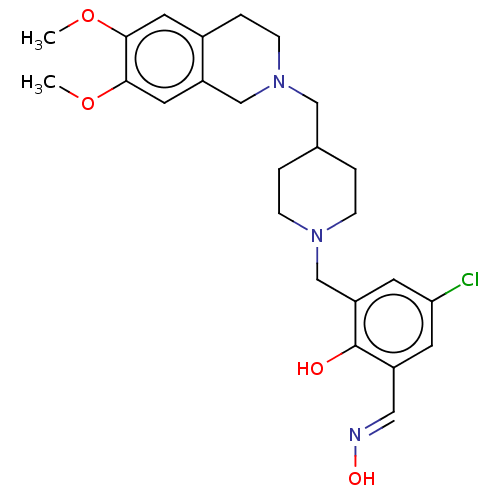
(CHEMBL4102122)Show SMILES COc1cc2CCN(CC3CCN(Cc4cc(Cl)cc(\C=N\O)c4O)CC3)Cc2cc1OC Show InChI InChI=1S/C25H32ClN3O4/c1-32-23-11-18-5-8-29(15-20(18)12-24(23)33-2)14-17-3-6-28(7-4-17)16-21-10-22(26)9-19(13-27-31)25(21)30/h9-13,17,30-31H,3-8,14-16H2,1-2H3/b27-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260310
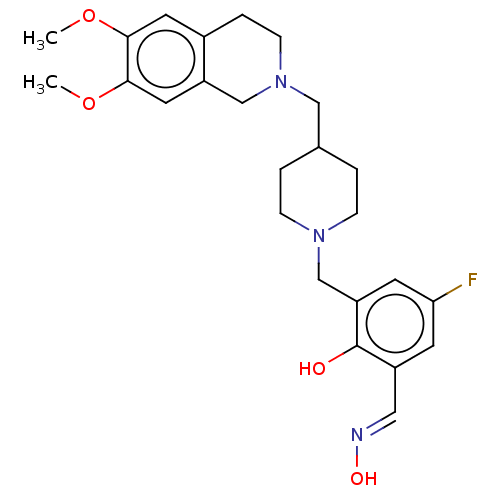
(CHEMBL4064154)Show SMILES COc1cc2CCN(CC3CCN(Cc4cc(F)cc(\C=N\O)c4O)CC3)Cc2cc1OC Show InChI InChI=1S/C25H32FN3O4/c1-32-23-11-18-5-8-29(15-20(18)12-24(23)33-2)14-17-3-6-28(7-4-17)16-21-10-22(26)9-19(13-27-31)25(21)30/h9-13,17,30-31H,3-8,14-16H2,1-2H3/b27-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50035852
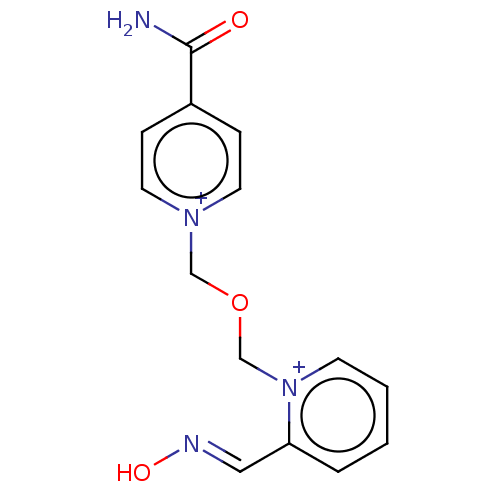
(HI-6)Show InChI InChI=1S/C14H14N4O3/c15-14(19)12-4-7-17(8-5-12)10-21-11-18-6-2-1-3-13(18)9-16-20/h1-9H,10-11H2,(H-,15,19)/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50260322
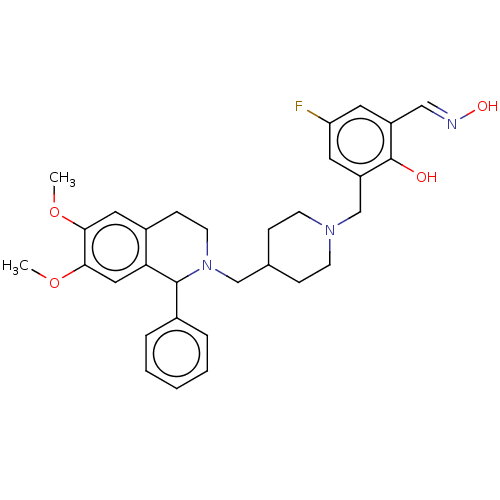
(CHEMBL4063109)Show SMILES COc1cc2CCN(CC3CCN(Cc4cc(F)cc(\C=N\O)c4O)CC3)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C31H36FN3O4/c1-38-28-16-23-10-13-35(30(22-6-4-3-5-7-22)27(23)17-29(28)39-2)19-21-8-11-34(12-9-21)20-25-15-26(32)14-24(18-33-37)31(25)36/h3-7,14-18,21,30,36-37H,8-13,19-20H2,1-2H3/b33-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fourth Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate preincubated for 30 mins followed by substrate addition measured every 5 mins for 20 mi... |
Bioorg Med Chem 25: 4497-4505 (2017)
Article DOI: 10.1016/j.bmc.2017.06.041
BindingDB Entry DOI: 10.7270/Q2SJ1P2T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data