

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492595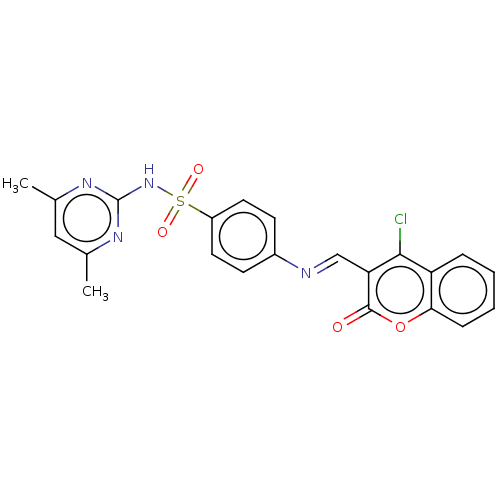 (CHEMBL2408077) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492590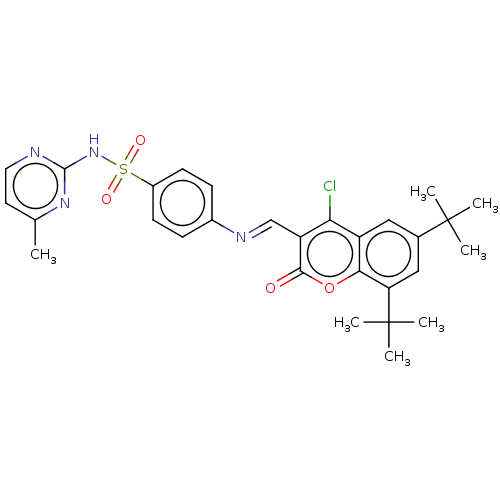 (CHEMBL2408085) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492592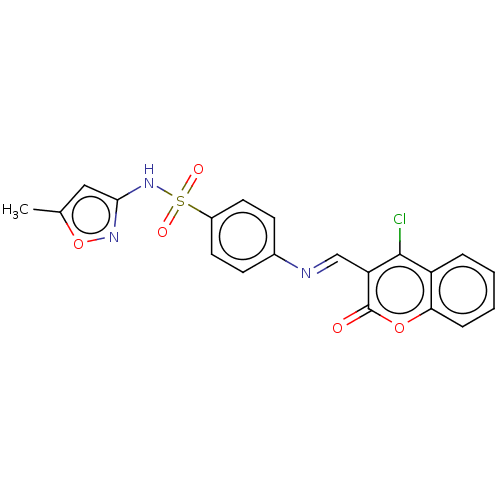 (CHEMBL2408078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant EGFR cytoplasmic domain (amino acids 645-1186) (unknown origin) expressed in Sf9 cells assessed as reducti... | Bioorg Med Chem Lett 24: 4472-6 (2014) Article DOI: 10.1016/j.bmcl.2014.07.094 BindingDB Entry DOI: 10.7270/Q2PV6N5T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492599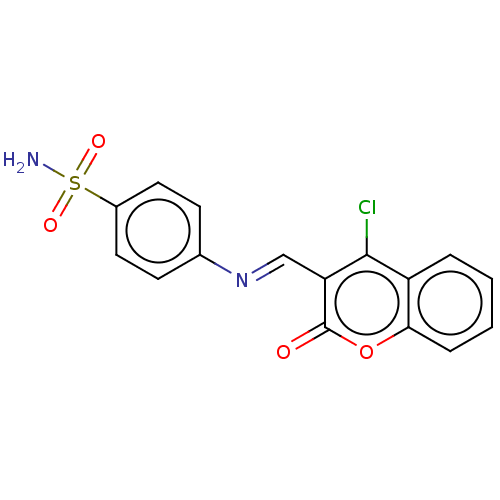 (CHEMBL2408074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492593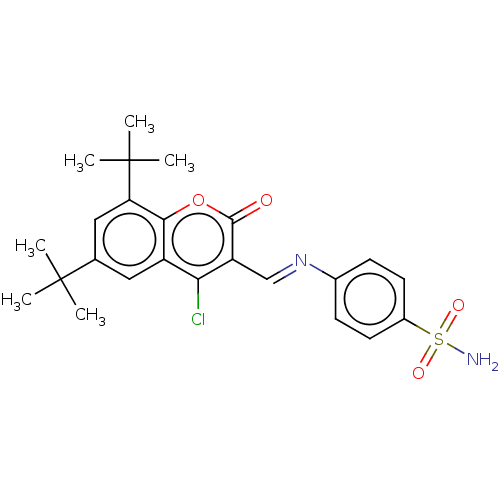 (CHEMBL2408080) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492588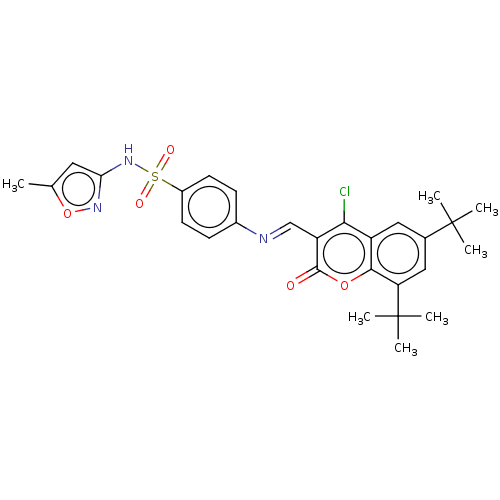 (CHEMBL2408084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492600 (CHEMBL2408073) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492598 (CHEMBL2408088) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492594 (CHEMBL2408079) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492597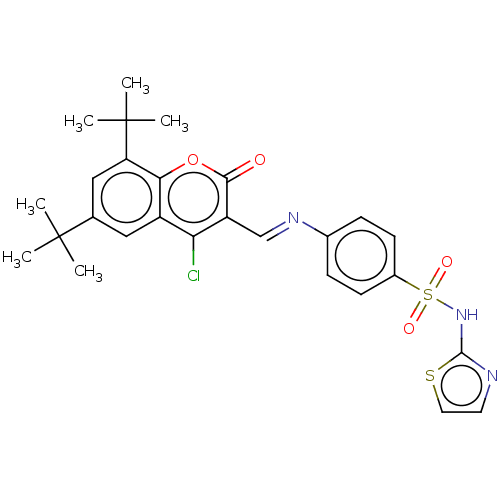 (CHEMBL2408081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492589 (CHEMBL2408090) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492596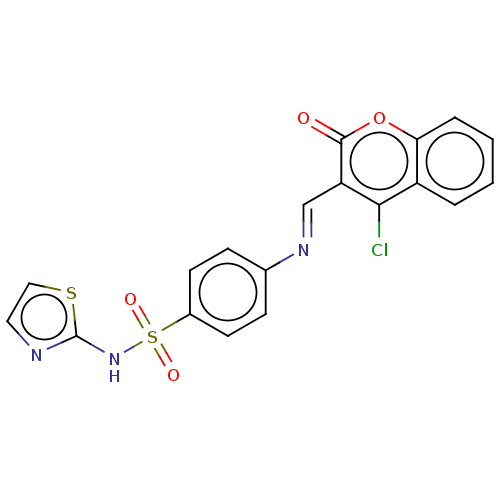 (CHEMBL2408075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492600 (CHEMBL2408073) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492590 (CHEMBL2408085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492601 (CHEMBL2408089) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492602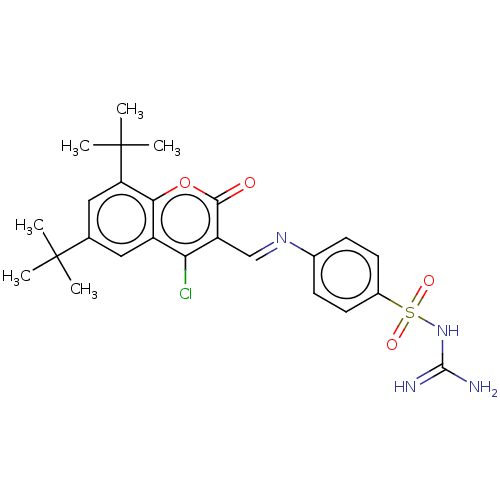 (CHEMBL2408082) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492594 (CHEMBL2408079) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492603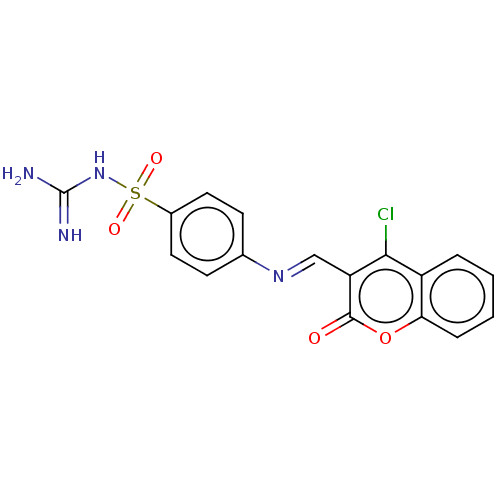 (CHEMBL2408076) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492587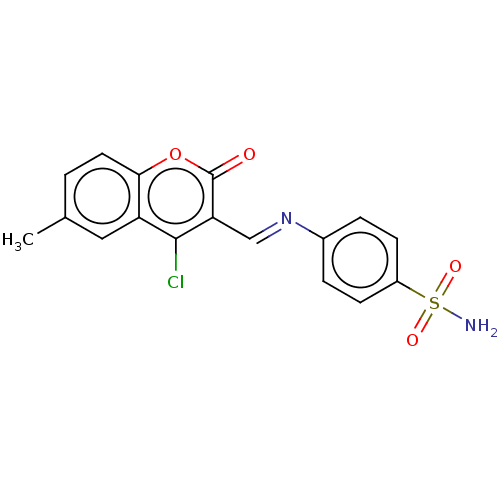 (CHEMBL2408086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492586 (CHEMBL2408087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492587 (CHEMBL2408086) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492591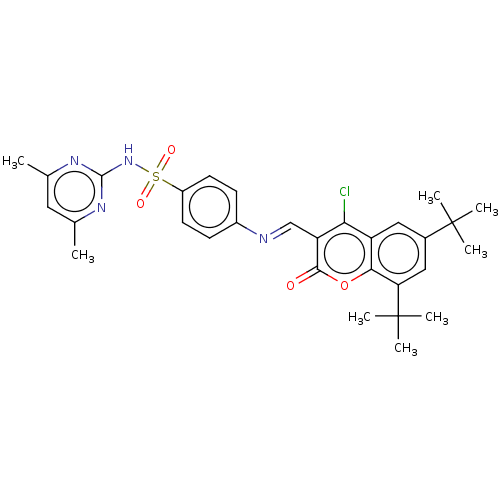 (CHEMBL2408083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50103527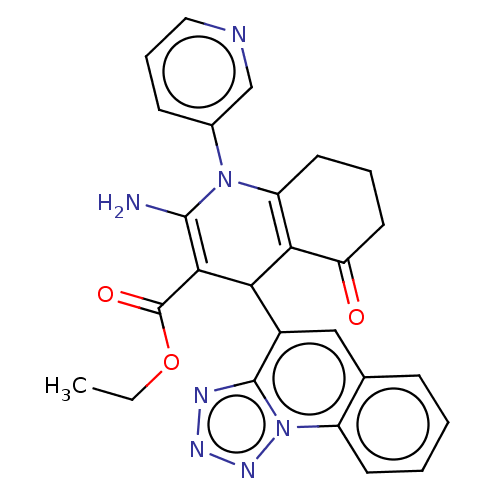 (CHEMBL3325477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant EGFR cytoplasmic domain (amino acids 645-1186) (unknown origin) expressed in Sf9 cells assessed as reducti... | Bioorg Med Chem Lett 24: 4472-6 (2014) Article DOI: 10.1016/j.bmcl.2014.07.094 BindingDB Entry DOI: 10.7270/Q2PV6N5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492599 (CHEMBL2408074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492598 (CHEMBL2408088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492602 (CHEMBL2408082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492593 (CHEMBL2408080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492586 (CHEMBL2408087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492597 (CHEMBL2408081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492595 (CHEMBL2408077) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492596 (CHEMBL2408075) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492592 (CHEMBL2408078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492601 (CHEMBL2408089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512017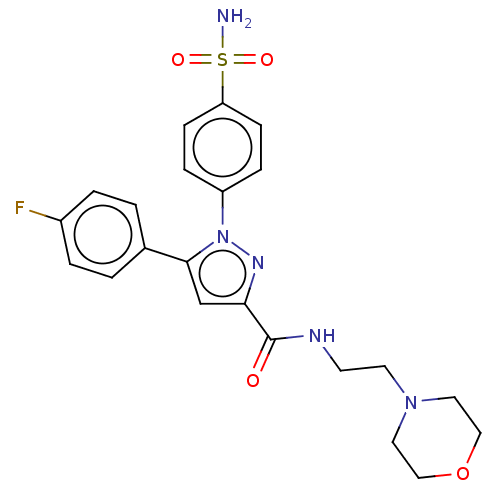 (CHEMBL4552101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512029 (CHEMBL4445481) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492591 (CHEMBL2408083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50492588 (CHEMBL2408084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492603 (CHEMBL2408076) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay | Eur J Med Chem 66: 1-11 (2013) Article DOI: 10.1016/j.ejmech.2013.04.035 BindingDB Entry DOI: 10.7270/Q2M61P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512044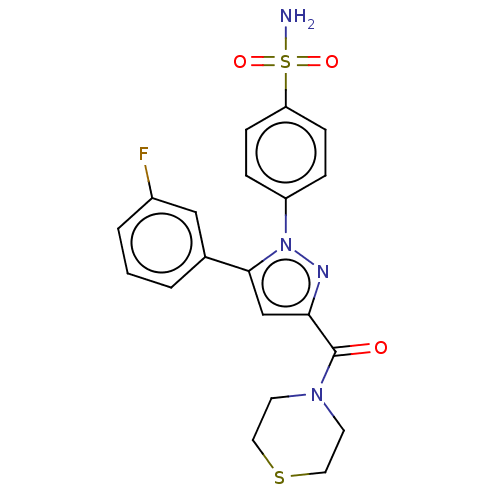 (CHEMBL4529375) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512028 (CHEMBL4462245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512024 (CHEMBL4476757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50102489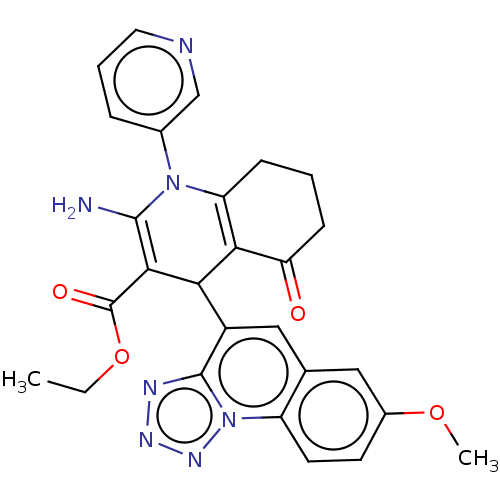 (CHEMBL3325479) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant EGFR cytoplasmic domain (amino acids 645-1186) (unknown origin) expressed in Sf9 cells assessed as reducti... | Bioorg Med Chem Lett 24: 4472-6 (2014) Article DOI: 10.1016/j.bmcl.2014.07.094 BindingDB Entry DOI: 10.7270/Q2PV6N5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50103527 (CHEMBL3325477) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant HER2 (unknown origin) expressed in Sf9 cells assessed as reduction in enzyme autophosphorylation by DELFIA... | Bioorg Med Chem Lett 24: 4472-6 (2014) Article DOI: 10.1016/j.bmcl.2014.07.094 BindingDB Entry DOI: 10.7270/Q2PV6N5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of His-tagged EGFR cytoplasmic domain (645-1186 aa) (unknown origin) assessed as inhibition of autophosphorylation by TR-fluorometry | Bioorg Med Chem Lett 26: 677-83 (2016) Article DOI: 10.1016/j.bmcl.2015.11.040 BindingDB Entry DOI: 10.7270/Q2K35WH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant HER2 (unknown origin) expressed in Sf9 cells assessed as reduction in enzyme autophosphorylation by DELFIA... | Bioorg Med Chem Lett 24: 4472-6 (2014) Article DOI: 10.1016/j.bmcl.2014.07.094 BindingDB Entry DOI: 10.7270/Q2PV6N5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512032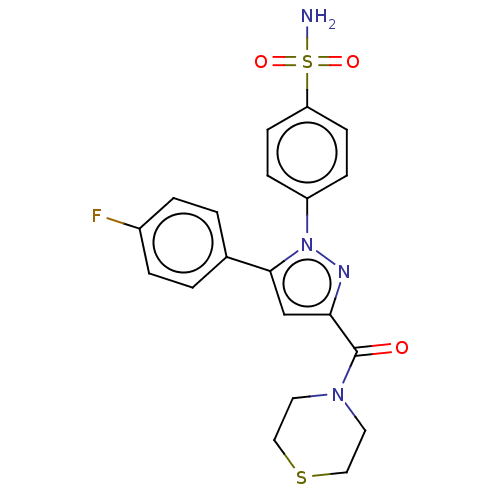 (CHEMBL4443996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50512023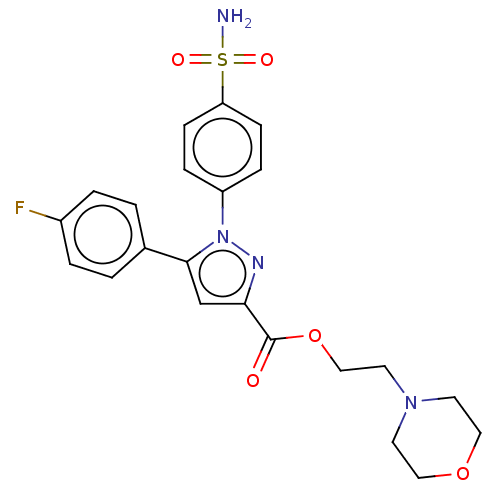 (CHEMBL4439374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate incubated for 10 mins by Ellman's reagent based COX-1/COX-2 ELISA assay | Eur J Med Chem 169: 168-184 (2019) Article DOI: 10.1016/j.ejmech.2019.03.008 BindingDB Entry DOI: 10.7270/Q2K077MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 353 total ) | Next | Last >> |