 Found 549 hits with Last Name = 'chu' and Initial = 'z'
Found 549 hits with Last Name = 'chu' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50343442

(CHEMBL1775179 | isopropyl 4-(1-(2-fluoro-4-(methyl...)Show SMILES CC(C)OC(=O)N1CCC(CC1)Oc1ncnc2n(ncc12)-c1ccc(cc1F)S(C)(=O)=O Show InChI InChI=1S/C21H24FN5O5S/c1-13(2)31-21(28)26-8-6-14(7-9-26)32-20-16-11-25-27(19(16)23-12-24-20)18-5-4-15(10-17(18)22)33(3,29)30/h4-5,10-14H,6-9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 3134-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.007
BindingDB Entry DOI: 10.7270/Q24T6JQF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50611953

(CHEMBL5289716)Show SMILES CN(CC1(CC1)C(=O)NC12CC3CC(C1)CC(C3)(C2)C(N)=O)S(=O)(=O)c1ccccc1F |TLB:17:16:10.11.12:14,19:16:10:12.13.14,19:16:10.11.12:14,THB:15:13:10:16.17.18,15:16:10:12.13.14,17:11:14:15.16.18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611950
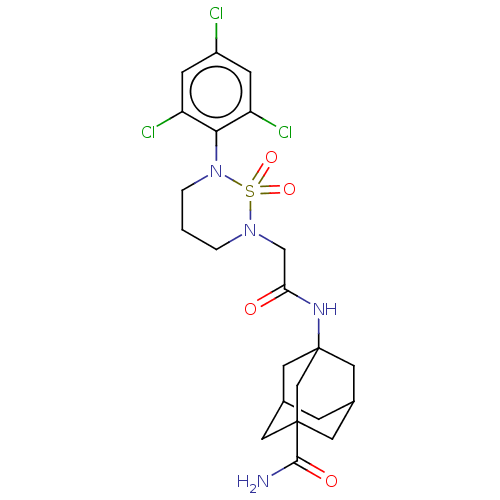
(CHEMBL5280689)Show SMILES NC(=O)C12CC3CC(CC(C3)(C1)NC(=O)CN1CCCN(c3c(Cl)cc(Cl)cc3Cl)S1(=O)=O)C2 |TLB:10:5:33:9.8.11,10:9:33:4.5.6,1:3:5.6.10:8,THB:4:3:5.6.10:8,4:5:8:33.3.11| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50568657
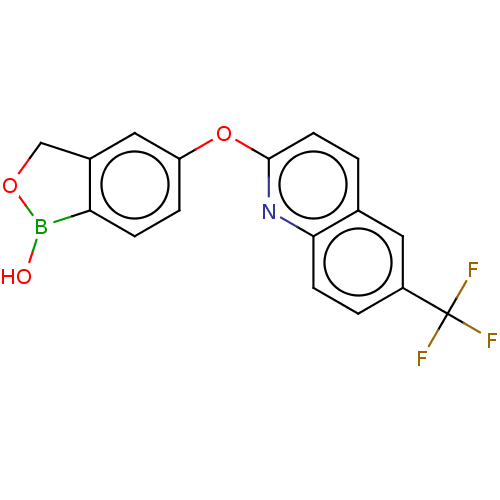
(CHEMBL4872870) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4B (unknown origin) assessed as hydrolysis of [3H]-cGMP into [3H]-GMP incubated for 30 mins by scintillation of proximity assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113171
BindingDB Entry DOI: 10.7270/Q2KW5KTJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520035
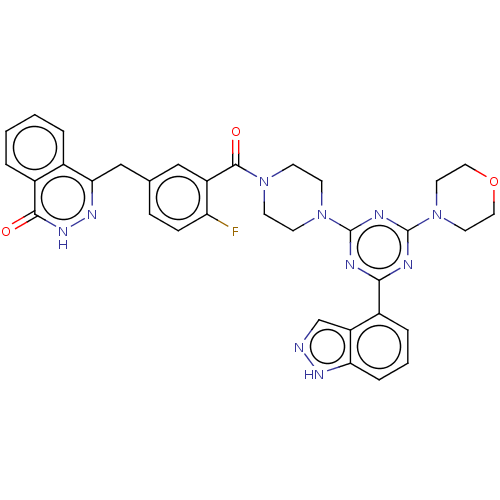
(CHEMBL4461392)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C34H31FN10O3/c35-27-9-8-21(19-29-22-4-1-2-5-24(22)31(46)42-41-29)18-25(27)32(47)43-10-12-44(13-11-43)33-37-30(23-6-3-7-28-26(23)20-36-40-28)38-34(39-33)45-14-16-48-17-15-45/h1-9,18,20H,10-17,19H2,(H,36,40)(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520036
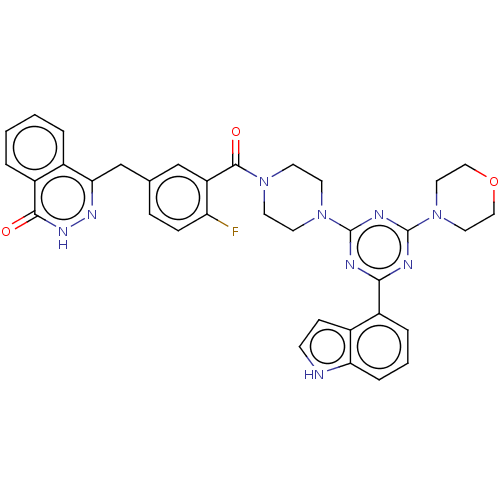
(CHEMBL4437593)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C35H32FN9O3/c36-28-9-8-22(21-30-23-4-1-2-5-26(23)32(46)42-41-30)20-27(28)33(47)43-12-14-44(15-13-43)34-38-31(25-6-3-7-29-24(25)10-11-37-29)39-35(40-34)45-16-18-48-19-17-45/h1-11,20,37H,12-19,21H2,(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520043

(CHEMBL4549595)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1ccc2cn[nH]c2c1)N1CCOCC1 Show InChI InChI=1S/C34H31FN10O3/c35-27-8-5-21(18-29-24-3-1-2-4-25(24)31(46)42-41-29)17-26(27)32(47)43-9-11-44(12-10-43)33-37-30(22-6-7-23-20-36-40-28(23)19-22)38-34(39-33)45-13-15-48-16-14-45/h1-8,17,19-20H,9-16,18H2,(H,36,40)(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520026

(CHEMBL4525998)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1ccc2[nH]ccc2c1)N1CCOCC1 Show InChI InChI=1S/C35H32FN9O3/c36-28-7-5-22(20-30-25-3-1-2-4-26(25)32(46)42-41-30)19-27(28)33(47)43-11-13-44(14-12-43)34-38-31(24-6-8-29-23(21-24)9-10-37-29)39-35(40-34)45-15-17-48-18-16-45/h1-10,19,21,37H,11-18,20H2,(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520031
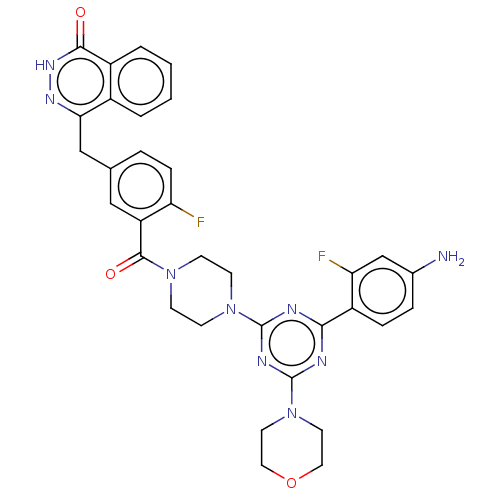
(CHEMBL4436980)Show SMILES Nc1ccc(c(F)c1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-26-8-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-25(26)31(46)42-9-11-43(12-10-42)32-37-29(24-7-6-21(36)19-27(24)35)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50353386

(CHEMBL1829763 | US8592410, 88 | US8592410, Compara...)Show SMILES C[C@H](N1CC[C@@](CCO)(OC1=O)c1ccc(F)cc1)c1ccc(cc1)-c1ccc(F)cc1F |r| Show InChI InChI=1S/C26H24F3NO3/c1-17(18-2-4-19(5-3-18)23-11-10-22(28)16-24(23)29)30-14-12-26(13-15-31,33-25(30)32)20-6-8-21(27)9-7-20/h2-11,16-17,31H,12-15H2,1H3/t17-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261
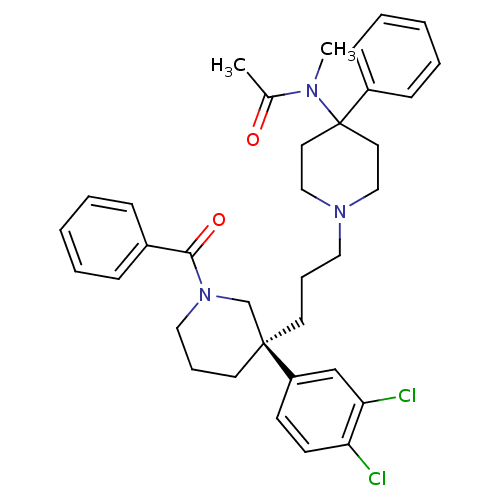
((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tachykinin receptor 3 binding affinity was determined by incubation with CHO cells expressing human NK3 receptors |
Bioorg Med Chem Lett 7: 555-560 (1997)
Article DOI: 10.1016/S0960-894X(97)00064-4
BindingDB Entry DOI: 10.7270/Q2MK6CX0 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520042

(CHEMBL4571423)Show SMILES Nc1ccc(cc1F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-25-7-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-24(25)31(46)42-9-11-43(12-10-42)32-37-29(21-6-8-27(36)26(35)19-21)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520033
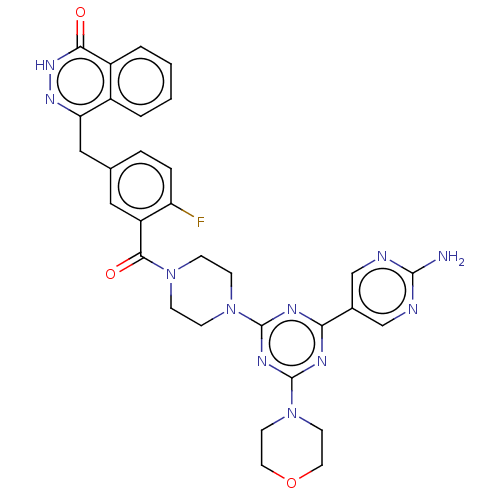
(CHEMBL4461382)Show SMILES Nc1ncc(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C31H30FN11O3/c32-24-6-5-19(16-25-21-3-1-2-4-22(21)27(44)40-39-25)15-23(24)28(45)41-7-9-42(10-8-41)30-36-26(20-17-34-29(33)35-18-20)37-31(38-30)43-11-13-46-14-12-43/h1-6,15,17-18H,7-14,16H2,(H,40,44)(H2,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611946

(HSD-016 | Hsd-016)Show SMILES C[C@@H]1CN(CCN1S(=O)(=O)c1cccc(c1)[C@@](C)(O)C(F)(F)F)c1ccc(F)cc1C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50611950

(CHEMBL5280689)Show SMILES NC(=O)C12CC3CC(CC(C3)(C1)NC(=O)CN1CCCN(c3c(Cl)cc(Cl)cc3Cl)S1(=O)=O)C2 |TLB:10:5:33:9.8.11,10:9:33:4.5.6,1:3:5.6.10:8,THB:4:3:5.6.10:8,4:5:8:33.3.11| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP2 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520032

(CHEMBL4551779)Show SMILES Nc1ccc(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C32H31FN10O3/c33-25-7-5-20(18-26-22-3-1-2-4-23(22)29(44)40-39-26)17-24(25)30(45)41-9-11-42(12-10-41)31-36-28(21-6-8-27(34)35-19-21)37-32(38-31)43-13-15-46-16-14-43/h1-8,17,19H,9-16,18H2,(H2,34,35)(H,40,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520041

(CHEMBL4446789)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H30F4N10O3/c34-25-6-5-19(16-26-21-3-1-2-4-22(21)29(48)44-43-26)15-23(25)30(49)45-7-9-46(10-8-45)31-40-28(41-32(42-31)47-11-13-50-14-12-47)20-17-24(33(35,36)37)27(38)39-18-20/h1-6,15,17-18H,7-14,16H2,(H2,38,39)(H,44,48) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520030
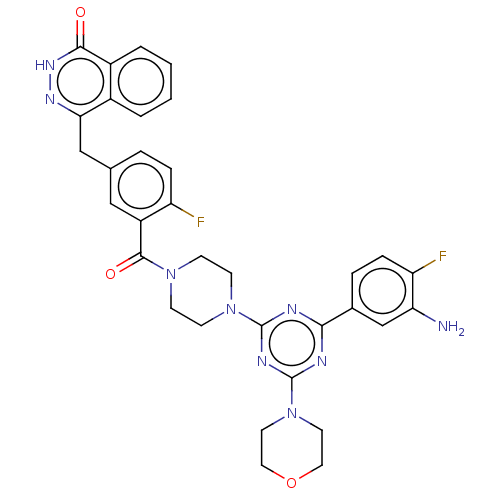
(CHEMBL4436366)Show SMILES Nc1cc(ccc1F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-25-7-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-24(25)31(46)42-9-11-43(12-10-42)32-37-29(21-6-8-26(35)27(36)19-21)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520027
(CHEMBL4438927)Show SMILES Nc1ccc(cc1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H32FN9O3/c34-27-10-5-21(20-28-24-3-1-2-4-25(24)30(44)40-39-28)19-26(27)31(45)41-11-13-42(14-12-41)32-36-29(22-6-8-23(35)9-7-22)37-33(38-32)43-15-17-46-18-16-43/h1-10,19H,11-18,20,35H2,(H,40,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01305
BindingDB Entry DOI: 10.7270/Q2M90DJF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50073179
(8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50587578
(CHEMBL5091825)Show SMILES OCCOCc1cn(CCNc2nonc2\C(Nc2ccc(F)c(Br)c2)=N\O)nn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation measured after 48 hrs by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01305
BindingDB Entry DOI: 10.7270/Q2M90DJF |
More data for this
Ligand-Target Pair | |
Hydroxysteroid 11-beta dehydrogenase 1
(Macaca mulatta) | BDBM50611953

(CHEMBL5289716)Show SMILES CN(CC1(CC1)C(=O)NC12CC3CC(C1)CC(C3)(C2)C(N)=O)S(=O)(=O)c1ccccc1F |TLB:17:16:10.11.12:14,19:16:10:12.13.14,19:16:10.11.12:14,THB:15:13:10:16.17.18,15:16:10:12.13.14,17:11:14:15.16.18| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520040

(CHEMBL4438741)Show SMILES Nc1ncc(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C31H28FN9O3/c32-24-6-5-18(14-25-20-3-1-2-4-21(20)29(42)39-38-25)13-23(24)30(43)41-8-7-22-26(17-41)36-27(19-15-34-31(33)35-16-19)37-28(22)40-9-11-44-12-10-40/h1-6,13,15-16H,7-12,14,17H2,(H,39,42)(H2,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611953

(CHEMBL5289716)Show SMILES CN(CC1(CC1)C(=O)NC12CC3CC(C1)CC(C3)(C2)C(N)=O)S(=O)(=O)c1ccccc1F |TLB:17:16:10.11.12:14,19:16:10:12.13.14,19:16:10.11.12:14,THB:15:13:10:16.17.18,15:16:10:12.13.14,17:11:14:15.16.18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529278
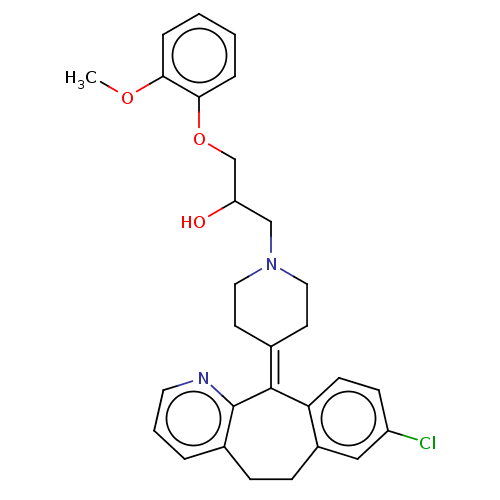
(CHEMBL4446961)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611951
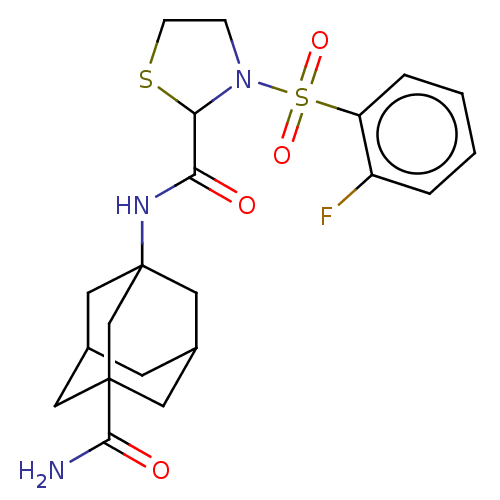
(CHEMBL5289930)Show SMILES NC(=O)C12CC3CC(CC(C3)(C1)NC(=O)C1SCCN1S(=O)(=O)c1ccccc1F)C2 |TLB:30:3:10:7.6.8,THB:4:5:3.11.30:8,4:3:10.5.6:8,1:3:10:7.6.8,1:3:10.5.6:8,30:7:10:4.3.11| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50568654

(CHEMBL4846842) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4B (unknown origin) assessed as hydrolysis of [3H]-cGMP into [3H]-GMP incubated for 30 mins by scintillation of proximity assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113171
BindingDB Entry DOI: 10.7270/Q2KW5KTJ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50568656

(CHEMBL4849717) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4B (unknown origin) assessed as hydrolysis of [3H]-cGMP into [3H]-GMP incubated for 30 mins by scintillation of proximity assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113171
BindingDB Entry DOI: 10.7270/Q2KW5KTJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP2 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529267

(CHEMBL4591702)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6@H](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 |r| Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM107664
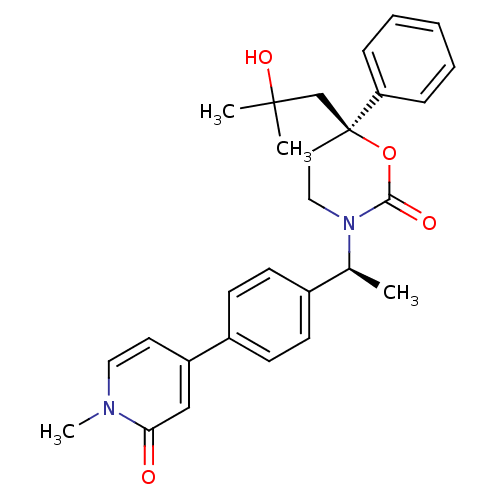
(US8575157, 48)Show SMILES C[C@H](N1CC[C@@](CC(C)(C)O)(OC1=O)c1ccccc1)c1ccc(cc1)-c1ccn(C)c(=O)c1 |r| Show InChI InChI=1S/C28H32N2O4/c1-20(21-10-12-22(13-11-21)23-14-16-29(4)25(31)18-23)30-17-15-28(34-26(30)32,19-27(2,3)33)24-8-6-5-7-9-24/h5-14,16,18,20,33H,15,17,19H2,1-4H3/t20-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529266
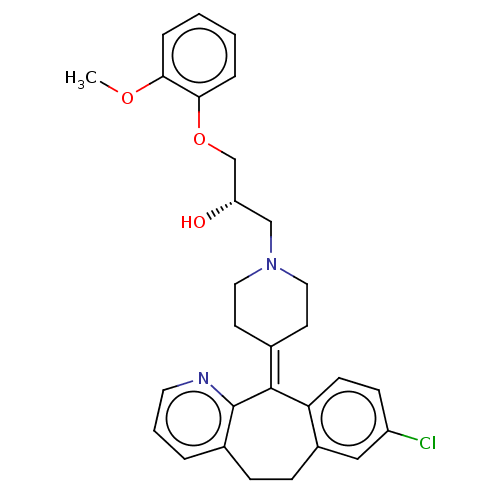
(CHEMBL4467816)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6@@H](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 |r| Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529273

(CHEMBL4556553)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1F)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H28ClFN2O2/c29-22-9-10-24-21(16-22)8-7-20-4-3-13-31-28(20)27(24)19-11-14-32(15-12-19)17-23(33)18-34-26-6-2-1-5-25(26)30/h1-6,9-10,13,16,23,33H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520040

(CHEMBL4438741)Show SMILES Nc1ncc(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C31H28FN9O3/c32-24-6-5-18(14-25-20-3-1-2-4-21(20)29(42)39-38-25)13-23(24)30(43)41-8-7-22-26(17-41)36-27(19-15-34-31(33)35-16-19)37-28(22)40-9-11-44-12-10-40/h1-6,13,15-16H,7-12,14,17H2,(H,39,42)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520029
(CHEMBL4593274)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2c(C1)nc(nc2N1CCOCC1)-c1cccc2[nH]ccc12 Show InChI InChI=1S/C35H30FN7O3/c36-28-9-8-21(19-30-22-4-1-2-5-25(22)34(44)41-40-30)18-27(28)35(45)43-13-11-26-31(20-43)38-32(39-33(26)42-14-16-46-17-15-42)24-6-3-7-29-23(24)10-12-37-29/h1-10,12,18,37H,11,13-17,19-20H2,(H,41,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611951

(CHEMBL5289930)Show SMILES NC(=O)C12CC3CC(CC(C3)(C1)NC(=O)C1SCCN1S(=O)(=O)c1ccccc1F)C2 |TLB:30:3:10:7.6.8,THB:4:5:3.11.30:8,4:3:10.5.6:8,1:3:10:7.6.8,1:3:10.5.6:8,30:7:10:4.3.11| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM107590

(US8575157, 31)Show SMILES C[C@H](N1CC[C@@](CCO)(OC1=O)c1ccc(F)cc1)c1ccc(cc1)-c1ccc(=O)n(C)c1 |r| Show InChI InChI=1S/C26H27FN2O4/c1-18(19-3-5-20(6-4-19)21-7-12-24(31)28(2)17-21)29-15-13-26(14-16-30,33-25(29)32)22-8-10-23(27)11-9-22/h3-12,17-18,30H,13-16H2,1-2H3/t18-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529276

(CHEMBL4587694)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H29ClN2O2/c29-23-10-11-26-22(17-23)9-8-21-5-4-14-30-28(21)27(26)20-12-15-31(16-13-20)18-24(32)19-33-25-6-2-1-3-7-25/h1-7,10-11,14,17,24,32H,8-9,12-13,15-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520039

(CHEMBL4531016)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2c(C1)nc(nc2N1CCOCC1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C34H29FN8O3/c35-27-9-8-20(17-29-21-4-1-2-5-23(21)33(44)41-40-29)16-25(27)34(45)43-11-10-24-30(19-43)37-31(38-32(24)42-12-14-46-15-13-42)22-6-3-7-28-26(22)18-36-39-28/h1-9,16,18H,10-15,17,19H2,(H,36,39)(H,41,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50587581
(CHEMBL5094068)Show SMILES CCOc1cc(CNC(=O)c2ncoc2-c2ccc(OC)cc2)cc(OCC)c1OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation measured after 48 hrs by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01305
BindingDB Entry DOI: 10.7270/Q2M90DJF |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50568655

(CHEMBL4877075) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4B (unknown origin) assessed as hydrolysis of [3H]-cGMP into [3H]-GMP incubated for 30 mins by scintillation of proximity assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113171
BindingDB Entry DOI: 10.7270/Q2KW5KTJ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50568649
(CHEMBL4865122) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4B (unknown origin) assessed as hydrolysis of [3H]-cGMP into [3H]-GMP incubated for 30 mins by scintillation of proximity assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113171
BindingDB Entry DOI: 10.7270/Q2KW5KTJ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562497
(CHEMBL4778760)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCc2c1)C(=O)C1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01305
BindingDB Entry DOI: 10.7270/Q2M90DJF |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50587583

(CHEMBL5071087)Show SMILES C[C@@]1(CC[C@H](O)CC1)[C@H](O)C[C@H]1c2c(cccc2F)-c2cncn12 |r,wU:1.0,4.4,wD:11.11,8.9,(10.47,-8.26,;10.88,-6.78,;12.02,-7.82,;13.48,-7.35,;13.81,-5.84,;15.27,-5.37,;12.66,-4.81,;11.19,-5.28,;9.41,-7.26,;9.09,-8.76,;8.27,-6.23,;6.8,-6.7,;5.55,-5.8,;4.31,-6.7,;2.91,-6.09,;2.75,-4.57,;3.99,-3.66,;5.39,-4.28,;6.64,-3.37,;4.78,-8.17,;4.31,-9.63,;5.55,-10.54,;6.8,-9.63,;6.32,-8.17,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01305
BindingDB Entry DOI: 10.7270/Q2M90DJF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 2 hrs by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data