

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50485906 (CHEMBL2180587) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485904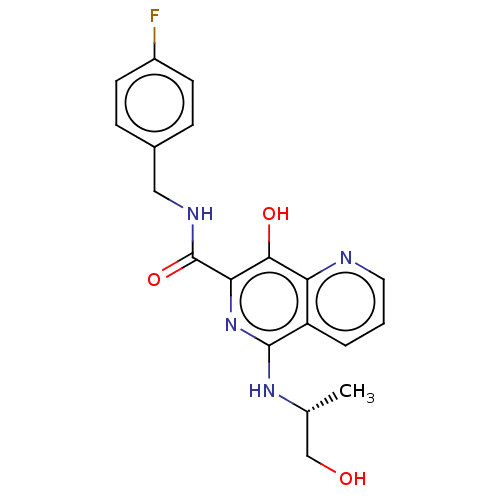 (CHEMBL2180591) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485905 (CHEMBL2180589) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50021581 (CHEMBL414850 | L-870810) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485914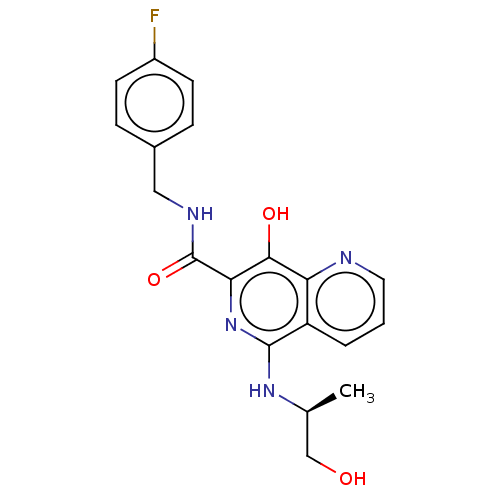 (CHEMBL2180590) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485907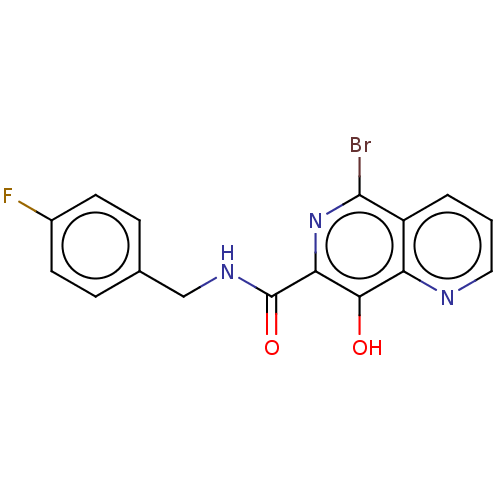 (CHEMBL2180599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485917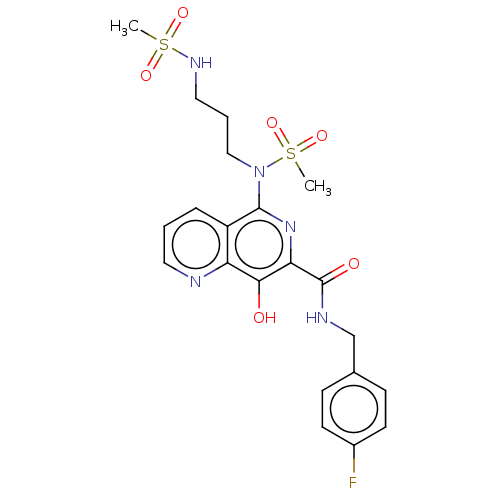 (CHEMBL2180585) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485913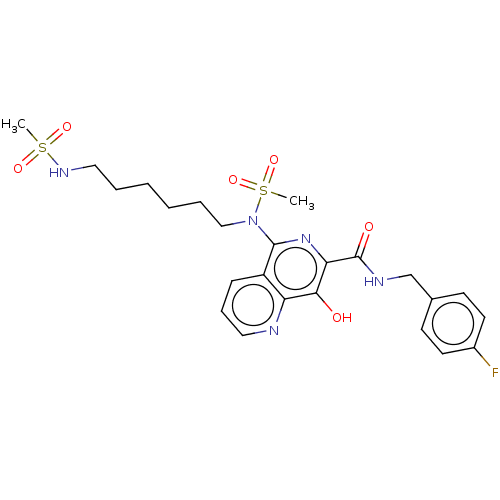 (CHEMBL2180586) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485911 (CHEMBL2180588) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042195 (CHEMBL3360188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042274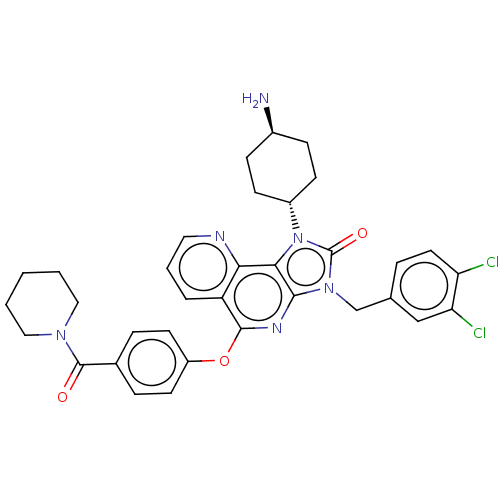 (CHEMBL3360682) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042189 (CHEMBL3360676) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042192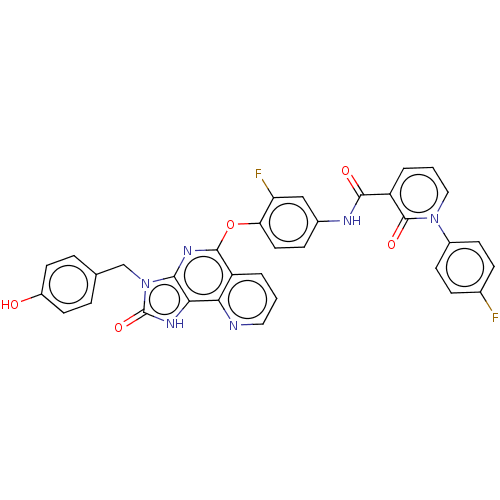 (CHEMBL3360673) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042272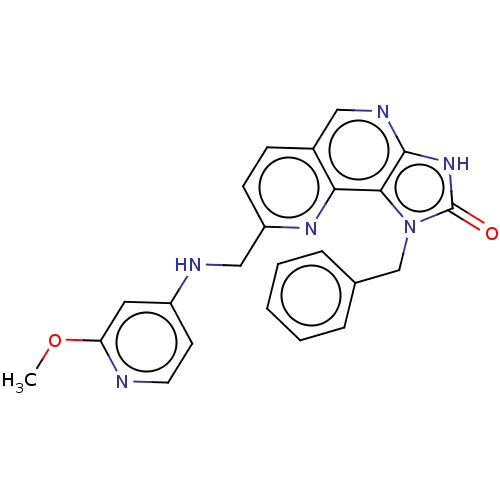 (CHEMBL3360181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492314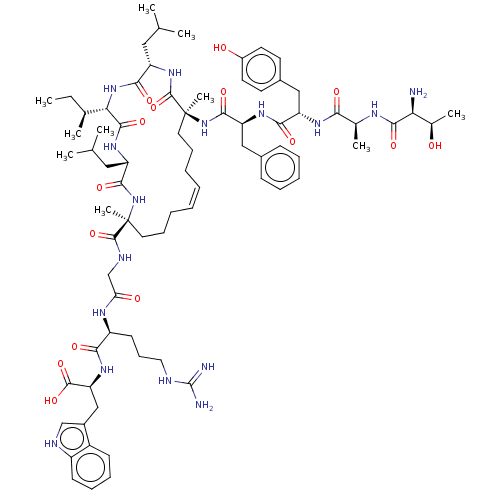 (CHEMBL2398137) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of HIV-1 His6-tagged integrase assessed as inhibition of LEDGF/p75-integrase interaction incubated 30 mins prior to flag-tagged LEDGF/p75 ... | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492308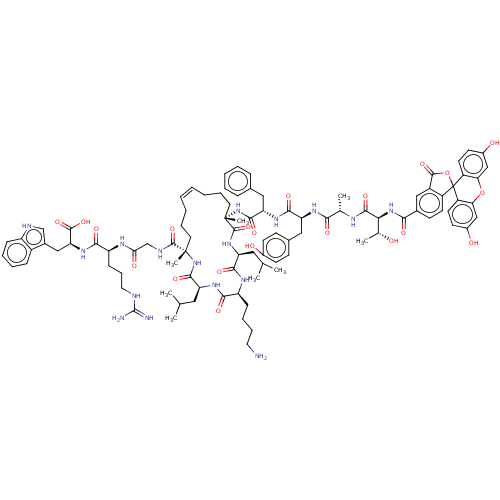 (CHEMBL2398138) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase 3' processing activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492308 (CHEMBL2398138) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492312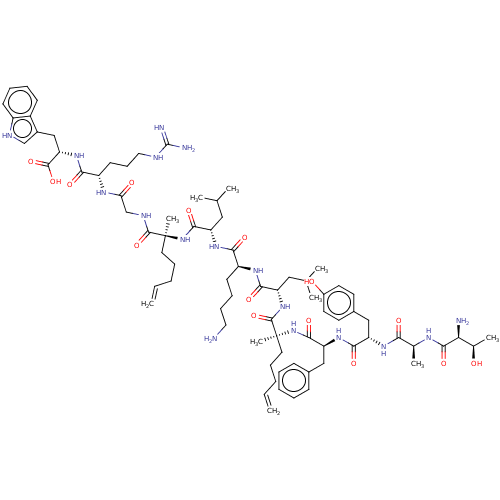 (CHEMBL2398127) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485906 (CHEMBL2180587) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492321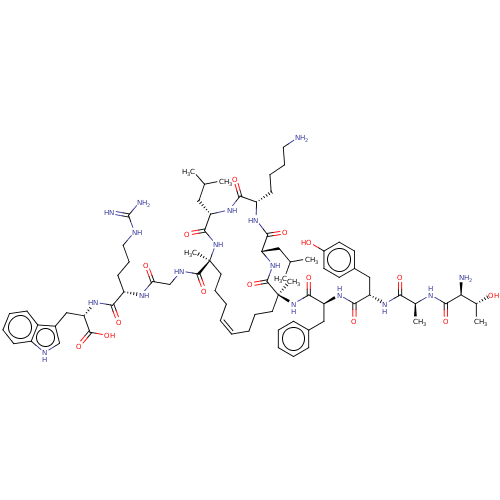 (CHEMBL2398128) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492318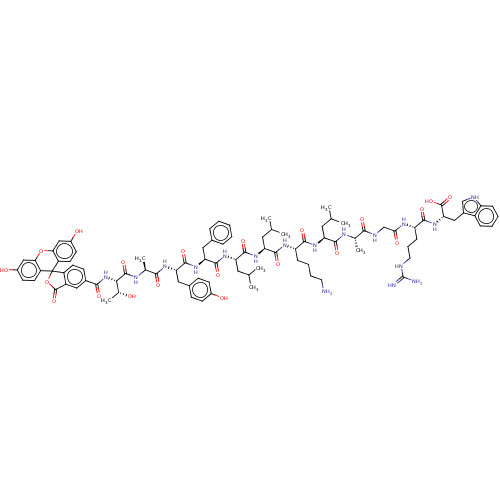 (CHEMBL2398124) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485904 (CHEMBL2180591) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485914 (CHEMBL2180590) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492312 (CHEMBL2398127) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase 3' processing activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492321 (CHEMBL2398128) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase 3' processing activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485905 (CHEMBL2180589) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50021581 (CHEMBL414850 | L-870810) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492309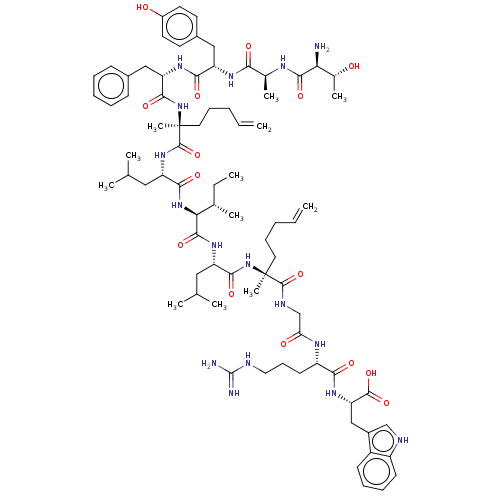 (CHEMBL2398136) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485913 (CHEMBL2180586) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042271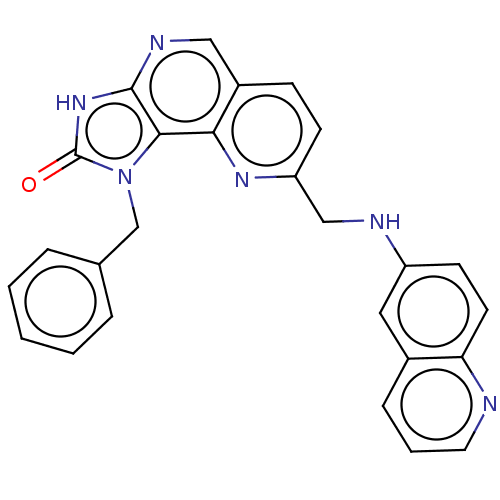 (CHEMBL3360183) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485912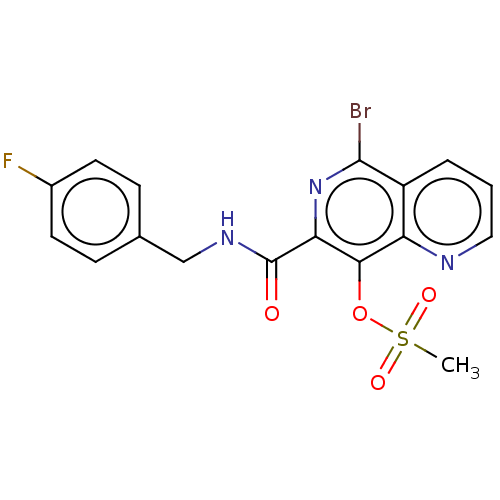 (CHEMBL2180600) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492310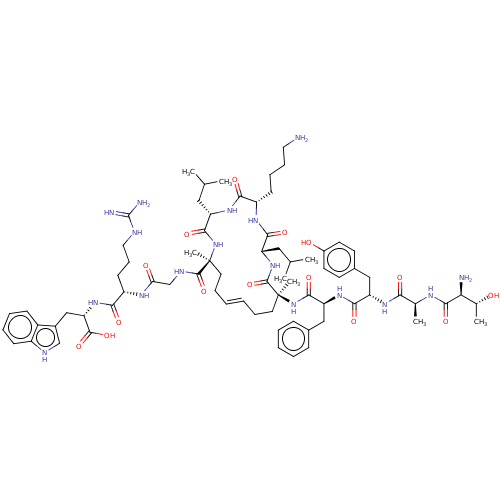 (CHEMBL2398133) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of HIV-1 His6-tagged integrase assessed as inhibition of LEDGF/p75-integrase interaction incubated 30 mins prior to flag-tagged LEDGF/p75 ... | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485907 (CHEMBL2180599) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50485911 (CHEMBL2180588) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate | J Med Chem 55: 9492-509 (2012) Article DOI: 10.1021/jm300667v BindingDB Entry DOI: 10.7270/Q2KS6VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492321 (CHEMBL2398128) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of HIV-1 His6-tagged integrase assessed as inhibition of LEDGF/p75-integrase interaction incubated 30 mins prior to flag-tagged LEDGF/p75 ... | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492312 (CHEMBL2398127) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of HIV-1 His6-tagged integrase assessed as inhibition of LEDGF/p75-integrase interaction incubated 30 mins prior to flag-tagged LEDGF/p75 ... | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492313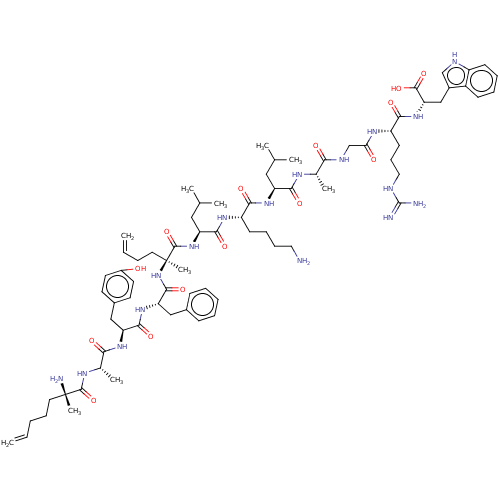 (CHEMBL2398125) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492309 (CHEMBL2398136) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase 3' processing activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492318 (CHEMBL2398124) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase 3' processing activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50492317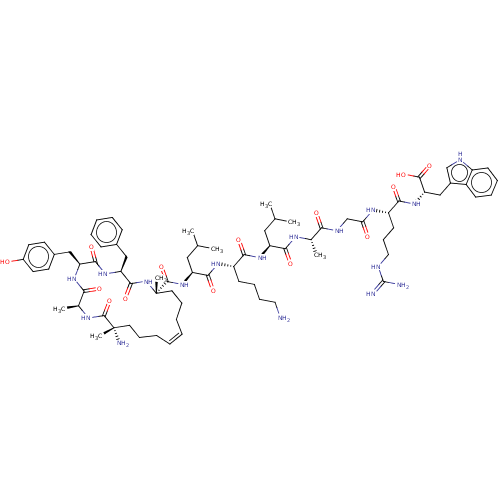 (CHEMBL2398126) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 integrase strand transfer activity after 30 mins | J Med Chem 56: 5601-12 (2013) Article DOI: 10.1021/jm4006516 BindingDB Entry DOI: 10.7270/Q2VX0KFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50042057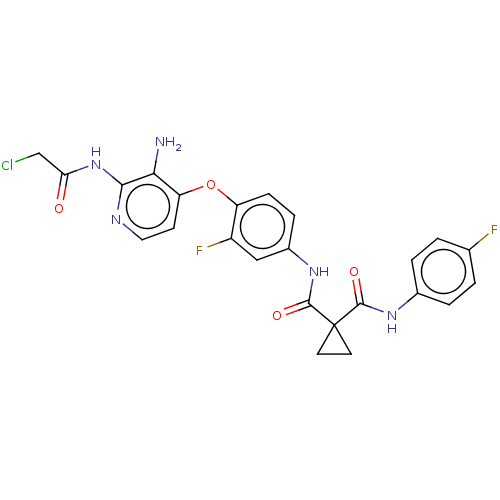 (CHEMBL3360679) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met (unknown origin) using poly (Glu,Tyr)4:1 substrate incubated for 60 mins by ELISA method | Bioorg Med Chem Lett 25: 708-16 (2015) Article DOI: 10.1016/j.bmcl.2014.11.070 BindingDB Entry DOI: 10.7270/Q21C1ZHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493201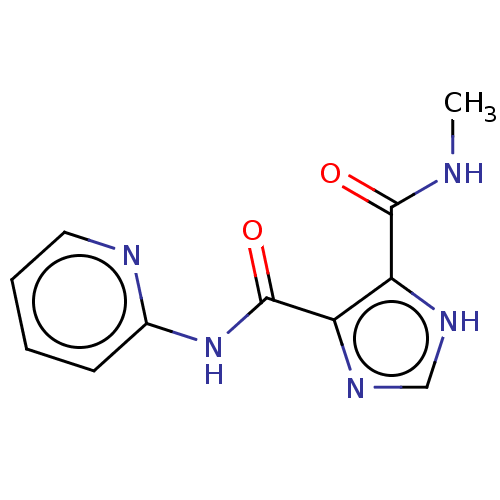 (CHEMBL1374702) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' strand transfer activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493202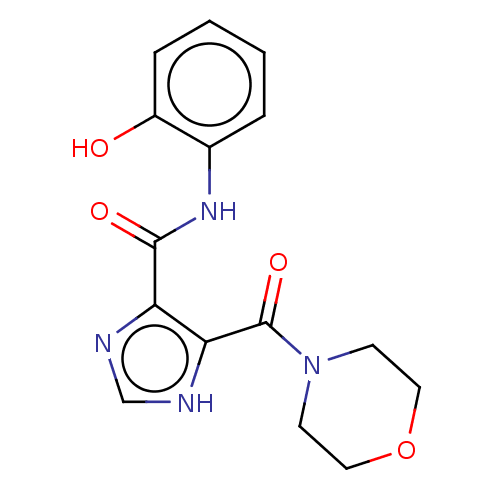 (CHEMBL2419618) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' strand transfer activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493203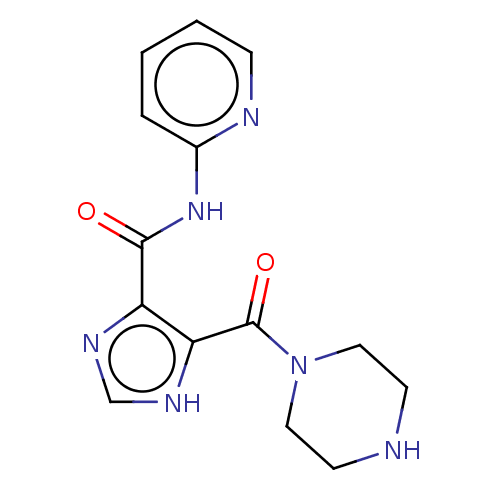 (CHEMBL2417573) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' strand transfer activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493204 (CHEMBL2419626) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' strand transfer activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493205 (CHEMBL2419623) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' strand transfer activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493206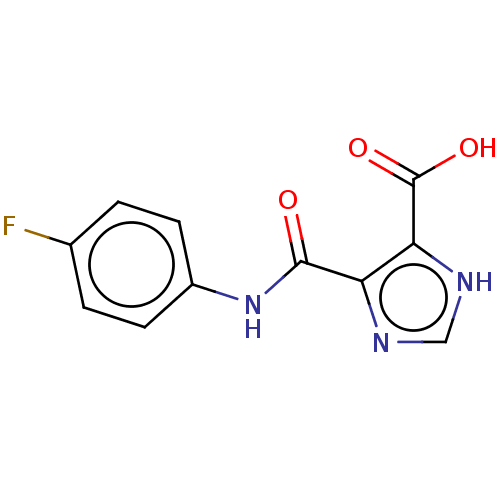 (CHEMBL1703922) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' strand transfer activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493201 (CHEMBL1374702) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' processing activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493207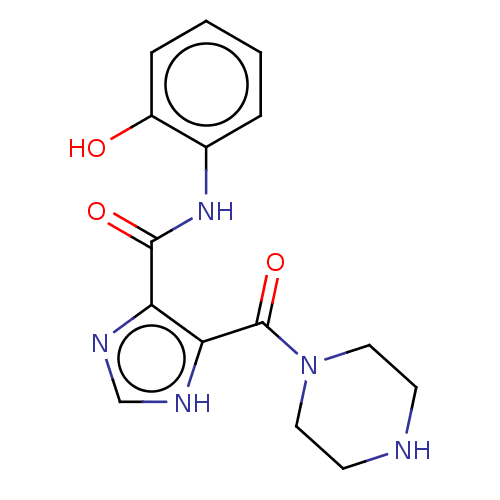 (CHEMBL2419620) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' processing activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50493208 (CHEMBL2419619) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged HIV-1 integrase 3' processing activity | Bioorg Med Chem 21: 5963-72 (2013) Article DOI: 10.1016/j.bmc.2013.07.047 BindingDB Entry DOI: 10.7270/Q2057JVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 124 total ) | Next | Last >> |