 Found 72 hits with Last Name = '''t hart' and Initial = 'p'
Found 72 hits with Last Name = '''t hart' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607731
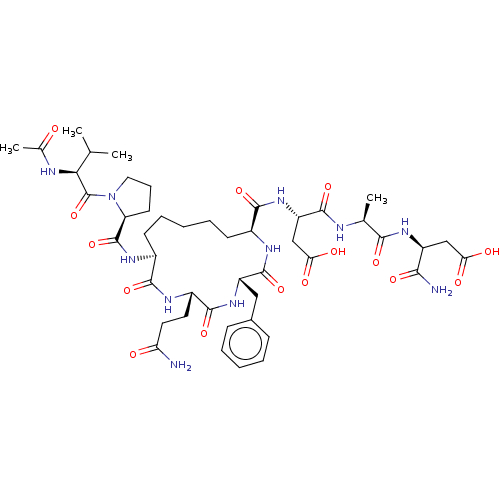
(CHEMBL5219517)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607732

(CHEMBL5220502)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 862 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607730
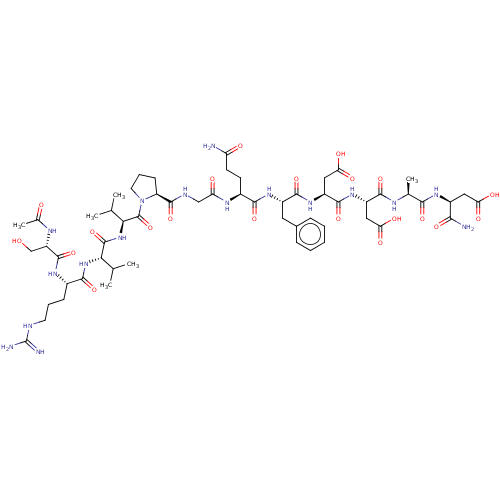
(CHEMBL5220691)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607728
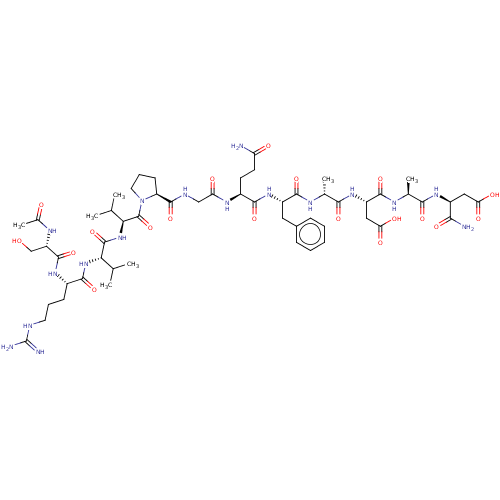
(CHEMBL5219427)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607727

(CHEMBL5219049)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607729
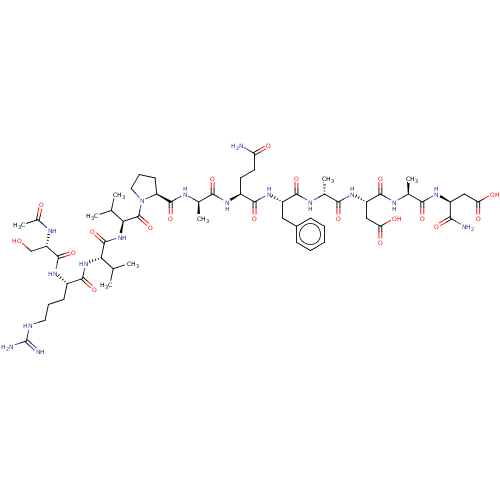
(CHEMBL5220586)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607733
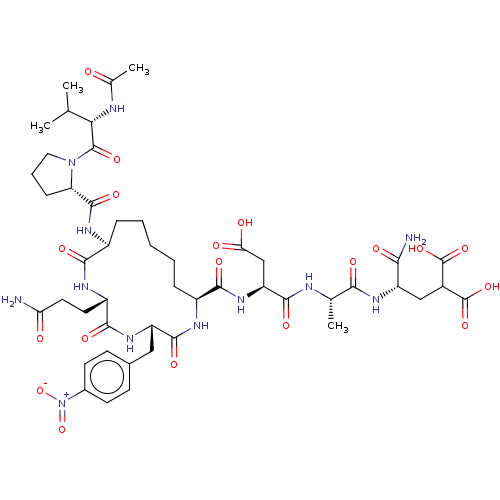
(CHEMBL5219642)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C(O)=O)C(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50028503
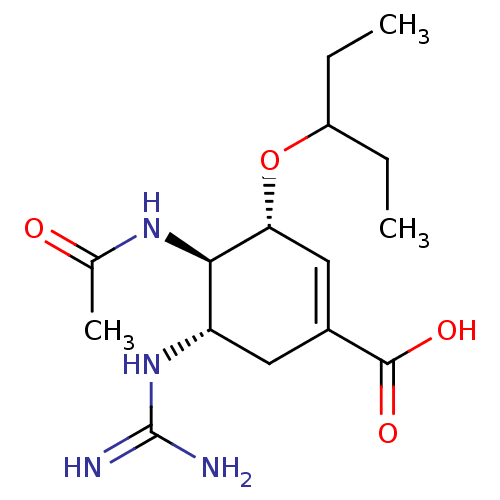
(CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h7,10-13H,4-6H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza virus neuraminidase |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50028503

(CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h7,10-13H,4-6H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50028503

(CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h7,10-13H,4-6H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496928
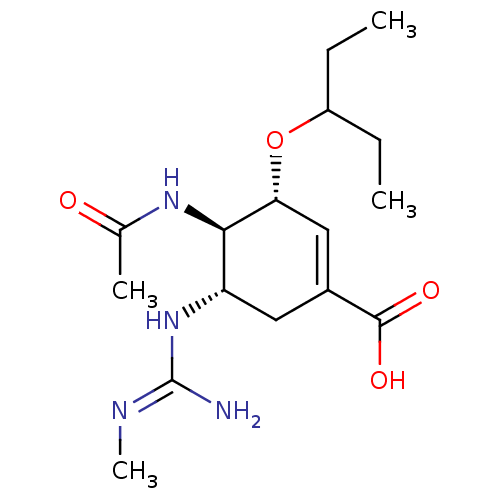
(CHEMBL3238015)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\C)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C16H28N4O4.C2HF3O2/c1-5-11(6-2)24-13-8-10(15(22)23)7-12(20-16(17)18-4)14(13)19-9(3)21;3-2(4,5)1(6)7/h8,11-14H,5-7H2,1-4H3,(H,19,21)(H,22,23)(H3,17,18,20);(H,6,7)/t12-,13+,14+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50028503

(CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h7,10-13H,4-6H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50028503

(CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h7,10-13H,4-6H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50496928

(CHEMBL3238015)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\C)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C16H28N4O4.C2HF3O2/c1-5-11(6-2)24-13-8-10(15(22)23)7-12(20-16(17)18-4)14(13)19-9(3)21;3-2(4,5)1(6)7/h8,11-14H,5-7H2,1-4H3,(H,19,21)(H,22,23)(H3,17,18,20);(H,6,7)/t12-,13+,14+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496926
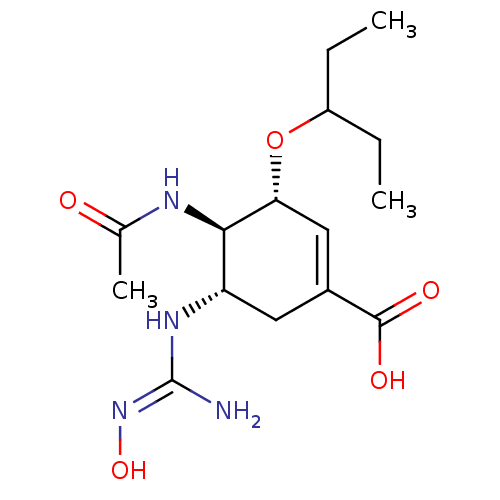
(CHEMBL3238021)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\O)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N4O5.C2HF3O2/c1-4-10(5-2)24-12-7-9(14(21)22)6-11(18-15(16)19-23)13(12)17-8(3)20;3-2(4,5)1(6)7/h7,10-13,23H,4-6H2,1-3H3,(H,17,20)(H,21,22)(H3,16,18,19);(H,6,7)/t11-,12+,13+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496928

(CHEMBL3238015)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\C)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C16H28N4O4.C2HF3O2/c1-5-11(6-2)24-13-8-10(15(22)23)7-12(20-16(17)18-4)14(13)19-9(3)21;3-2(4,5)1(6)7/h8,11-14H,5-7H2,1-4H3,(H,19,21)(H,22,23)(H3,17,18,20);(H,6,7)/t12-,13+,14+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496928

(CHEMBL3238015)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\C)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C16H28N4O4.C2HF3O2/c1-5-11(6-2)24-13-8-10(15(22)23)7-12(20-16(17)18-4)14(13)19-9(3)21;3-2(4,5)1(6)7/h8,11-14H,5-7H2,1-4H3,(H,19,21)(H,22,23)(H3,17,18,20);(H,6,7)/t12-,13+,14+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496927
(CHEMBL3238022)Show SMILES CC(=O)N[C@@H]1[C@@H](NC(=N)NC(=O)CSc2ccc3ccccc3c2)C=C(OC1[C@H](O)[C@H](O)CO)C(O)=O |r,c:26| Show InChI InChI=1S/C24H28N4O8S/c1-12(30)26-20-16(9-18(23(34)35)36-22(20)21(33)17(31)10-29)27-24(25)28-19(32)11-37-15-7-6-13-4-2-3-5-14(13)8-15/h2-9,16-17,20-22,29,31,33H,10-11H2,1H3,(H,26,30)(H,34,35)(H3,25,27,28,32)/t16-,17+,20+,21+,22?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus SN33 H1N1 neuraminidase using MU-NANA as substrate after 1 hr by spectrofluorometric analysis |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496926

(CHEMBL3238021)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\O)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N4O5.C2HF3O2/c1-4-10(5-2)24-12-7-9(14(21)22)6-11(18-15(16)19-23)13(12)17-8(3)20;3-2(4,5)1(6)7/h7,10-13,23H,4-6H2,1-3H3,(H,17,20)(H,21,22)(H3,16,18,19);(H,6,7)/t11-,12+,13+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496926

(CHEMBL3238021)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\O)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N4O5.C2HF3O2/c1-4-10(5-2)24-12-7-9(14(21)22)6-11(18-15(16)19-23)13(12)17-8(3)20;3-2(4,5)1(6)7/h7,10-13,23H,4-6H2,1-3H3,(H,17,20)(H,21,22)(H3,16,18,19);(H,6,7)/t11-,12+,13+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50496926

(CHEMBL3238021)Show SMILES OC(=O)C(F)(F)F.CCC(CC)O[C@@H]1C=C(C[C@H](N\C(N)=N\O)[C@H]1NC(C)=O)C(O)=O |r,c:13| Show InChI InChI=1S/C15H26N4O5.C2HF3O2/c1-4-10(5-2)24-12-7-9(14(21)22)6-11(18-15(16)19-23)13(12)17-8(3)20;3-2(4,5)1(6)7/h7,10-13,23H,4-6H2,1-3H3,(H,17,20)(H,21,22)(H3,16,18,19);(H,6,7)/t11-,12+,13+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) wild-type neuraminidase using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase H274Y mutant using MU-NANA as substrate after 1 hr |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607731

(CHEMBL5219517)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607731

(CHEMBL5219517)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607732

(CHEMBL5220502)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607732

(CHEMBL5220502)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607733

(CHEMBL5219642)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C(O)=O)C(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607726
(CHEMBL5219864)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC1=O)C(=O)N[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@@H](CC(C(O)=O)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607687
(CHEMBL5218659)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607688
(CHEMBL5219345)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CNC(=O)CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607689
(CHEMBL5218923)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCNC(=O)C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607690
(CHEMBL5220982)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCNC(=O)CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607691

(CHEMBL5220153)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CNC(=O)C[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607692
(CHEMBL5220599)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CNC(=O)CC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607693
(CHEMBL5220557)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCNC(=O)C[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607694

(CHEMBL5220853)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCNC(=O)CC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607695
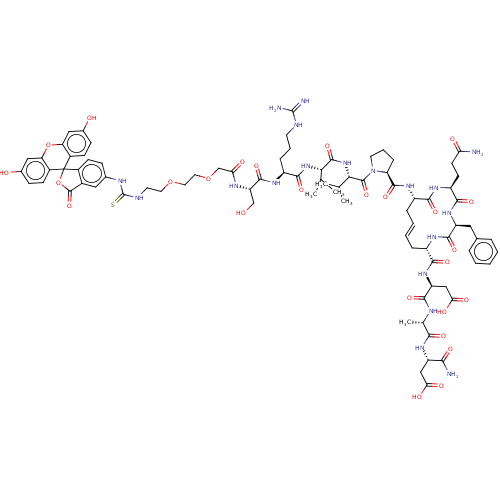
(CHEMBL5218466)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1C\C=C\C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r,t:85| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 719 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607696

(CHEMBL5219439)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607697

(CHEMBL5218911)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1C\C=C\C[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r,t:85| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607697

(CHEMBL5218911)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1C\C=C\C[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r,t:85| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607698
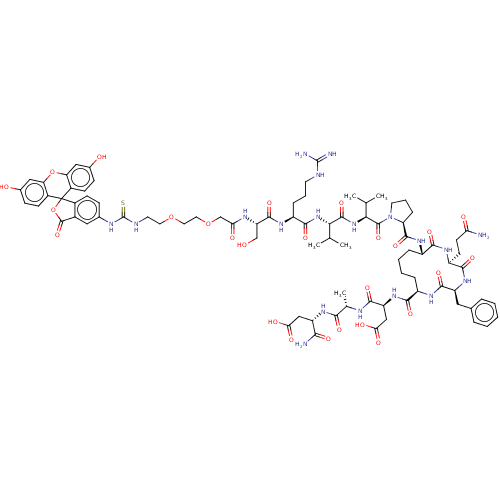
(CHEMBL5220001)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)COCCOCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607699
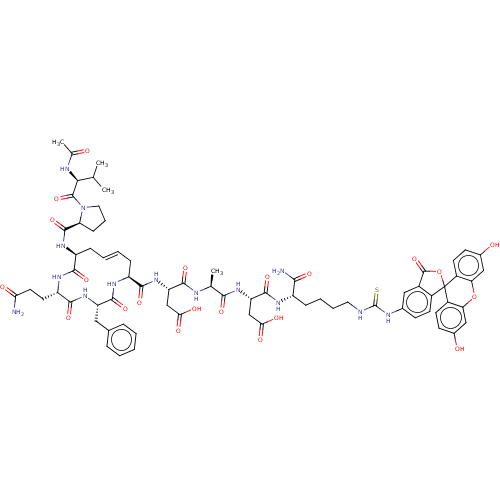
(CHEMBL5219742)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1C\C=C\C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r,t:21| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
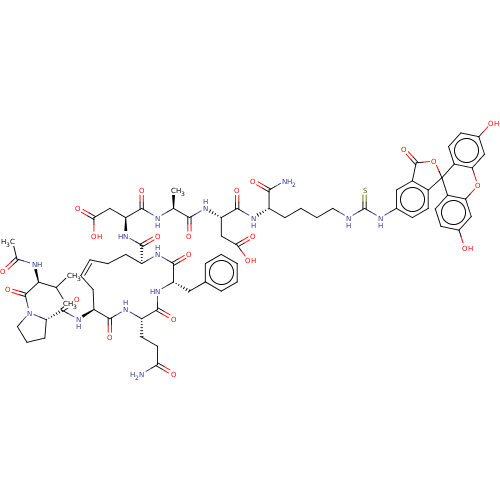
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607700
(CHEMBL5220186)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1C\C=C/CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r,c:21| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607700

(CHEMBL5220186)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1C\C=C/CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r,c:21| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607701
(CHEMBL5219207)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CC\C=C\C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r,t:22| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607702
(CHEMBL5219972)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CC\C=C\CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r,t:22| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |
Methylosome protein 50/Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50607703
(CHEMBL5218498)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CCCCC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01273
BindingDB Entry DOI: 10.7270/Q2VD73K1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data