 Found 16 hits with Last Name = 'abonia' and Initial = 'r'
Found 16 hits with Last Name = 'abonia' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50398064

(CHEMBL2181927)Show SMILES CCOc1ncc(C)c2N=C(C)C([C@@H](c3ccc(cc3OC)C#N)c12)C(N)=O |r,t:9| Show InChI InChI=1S/C21H22N4O3/c1-5-28-21-18-17(14-7-6-13(9-22)8-15(14)27-4)16(20(23)26)12(3)25-19(18)11(2)10-24-21/h6-8,10,16-17H,5H2,1-4H3,(H2,23,26)/t16?,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad del Valle
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor (unknown origin) |
Eur J Med Chem 60: 1-9 (2013)
Article DOI: 10.1016/j.ejmech.2012.11.037
BindingDB Entry DOI: 10.7270/Q29888B0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) using arachidonic acid as substrate preincubated for 20 mins followed by substrate addition measured at 1 sec int... |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM198173
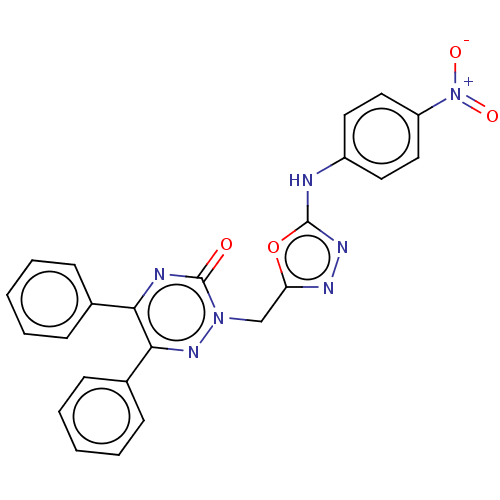
(2-((5-(4-Nitrophenylamino)-1,3,4-oxadiazol-2-yl)me...)Show SMILES [O-][N+](=O)c1ccc(Nc2nnc(Cn3nc(-c4ccccc4)c(nc3=O)-c3ccccc3)o2)cc1 Show InChI InChI=1S/C24H17N7O4/c32-24-26-21(16-7-3-1-4-8-16)22(17-9-5-2-6-10-17)29-30(24)15-20-27-28-23(35-20)25-18-11-13-19(14-12-18)31(33)34/h1-14H,15H2,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50507265

(CHEMBL4536074)Show SMILES O=c1nc(-c2ccccc2)c(nn1Cc1nnc(Nc2ccccc2)o1)-c1ccccc1 Show InChI InChI=1S/C24H18N6O2/c31-24-26-21(17-10-4-1-5-11-17)22(18-12-6-2-7-13-18)29-30(24)16-20-27-28-23(32-20)25-19-14-8-3-9-15-19/h1-15H,16H2,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM198171

(2-((5-(4-Methoxyphenylamino)-1,3,4-oxadiazol-2-yl)...)Show SMILES COc1ccc(Nc2nnc(Cn3nc(-c4ccccc4)c(nc3=O)-c3ccccc3)o2)cc1 Show InChI InChI=1S/C25H20N6O3/c1-33-20-14-12-19(13-15-20)26-24-29-28-21(34-24)16-31-25(32)27-22(17-8-4-2-5-9-17)23(30-31)18-10-6-3-7-11-18/h2-15H,16H2,1H3,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM198172
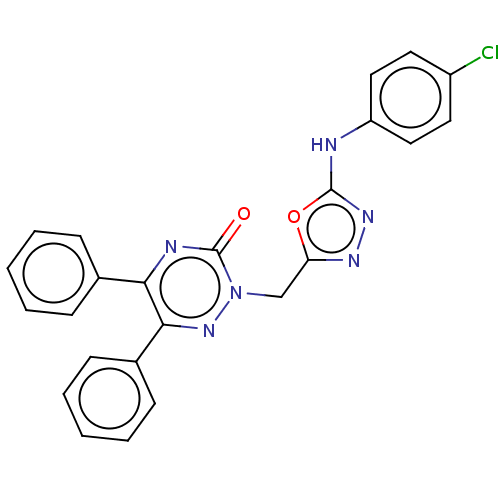
(2-((5-(4-Chlorophenylamino)-1,3,4-oxadiazol-2-yl)m...)Show SMILES Clc1ccc(Nc2nnc(Cn3nc(-c4ccccc4)c(nc3=O)-c3ccccc3)o2)cc1 Show InChI InChI=1S/C24H17ClN6O2/c25-18-11-13-19(14-12-18)26-23-29-28-20(33-23)15-31-24(32)27-21(16-7-3-1-4-8-16)22(30-31)17-9-5-2-6-10-17/h1-14H,15H2,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50507267
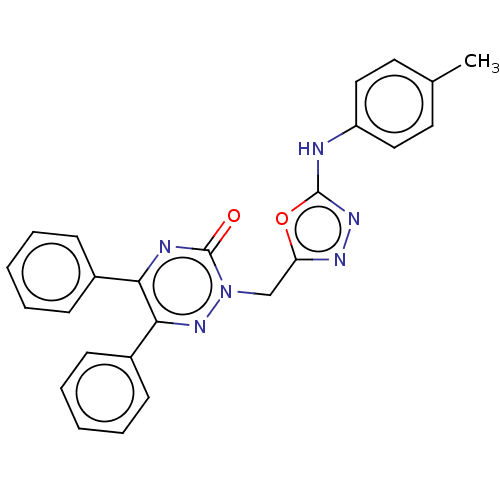
(CHEMBL4444061)Show SMILES Cc1ccc(Nc2nnc(Cn3nc(-c4ccccc4)c(nc3=O)-c3ccccc3)o2)cc1 Show InChI InChI=1S/C25H20N6O2/c1-17-12-14-20(15-13-17)26-24-29-28-21(33-24)16-31-25(32)27-22(18-8-4-2-5-9-18)23(30-31)19-10-6-3-7-11-19/h2-15H,16H2,1H3,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as decrease in PGE2 release after 10 mins by ELISA |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by colorimetric assay |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507266
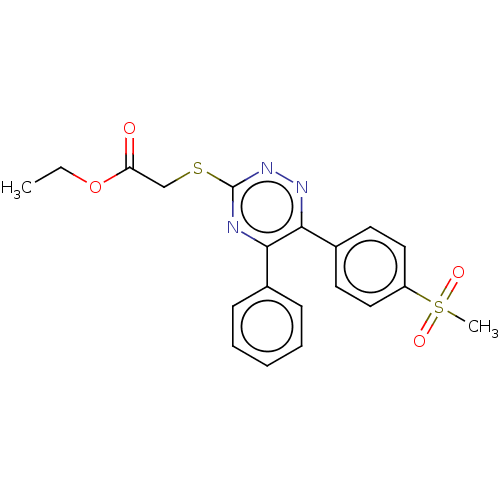
(CHEMBL4555690)Show SMILES CCOC(=O)CSc1nnc(-c2ccc(cc2)S(C)(=O)=O)c(n1)-c1ccccc1 Show InChI InChI=1S/C20H19N3O4S2/c1-3-27-17(24)13-28-20-21-18(14-7-5-4-6-8-14)19(22-23-20)15-9-11-16(12-10-15)29(2,25)26/h4-12H,3,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as decrease in PGE2 release after 10 mins by ELISA |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507268
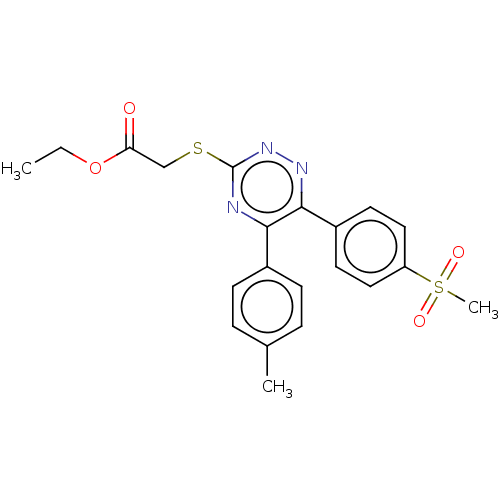
(CHEMBL4456497)Show SMILES CCOC(=O)CSc1nnc(-c2ccc(cc2)S(C)(=O)=O)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C21H21N3O4S2/c1-4-28-18(25)13-29-21-22-19(15-7-5-14(2)6-8-15)20(23-24-21)16-9-11-17(12-10-16)30(3,26)27/h5-12H,4,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as decrease in PGE2 release after 10 mins by ELISA |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507263

(CHEMBL4474936)Show SMILES CCOC(=O)CSc1nnc(-c2ccc(cc2)S(C)(=O)=O)c(n1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H18ClN3O4S2/c1-3-28-17(25)12-29-20-22-18(13-4-8-15(21)9-5-13)19(23-24-20)14-6-10-16(11-7-14)30(2,26)27/h4-11H,3,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as decrease in PGE2 release after 10 mins by ELISA |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507262
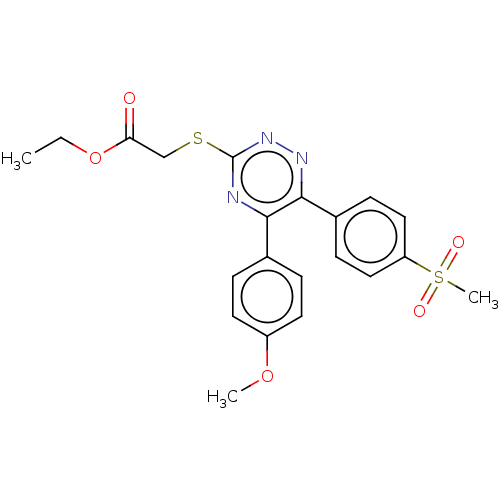
(CHEMBL4443940)Show SMILES CCOC(=O)CSc1nnc(-c2ccc(cc2)S(C)(=O)=O)c(n1)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H21N3O5S2/c1-4-29-18(25)13-30-21-22-19(14-5-9-16(28-2)10-6-14)20(23-24-21)15-7-11-17(12-8-15)31(3,26)27/h5-12H,4,13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as decrease in PGE2 release after 10 mins by ELISA |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507264

(CHEMBL4466839)Show SMILES CCOC(=O)CSc1nnc(-c2ccc(cc2)S(C)(=O)=O)c(n1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H18FN3O4S2/c1-3-28-17(25)12-29-20-22-18(13-4-8-15(21)9-5-13)19(23-24-20)14-6-10-16(11-7-14)30(2,26)27/h4-11H,3,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as decrease in PGE2 release after 10 mins by ELISA |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507261
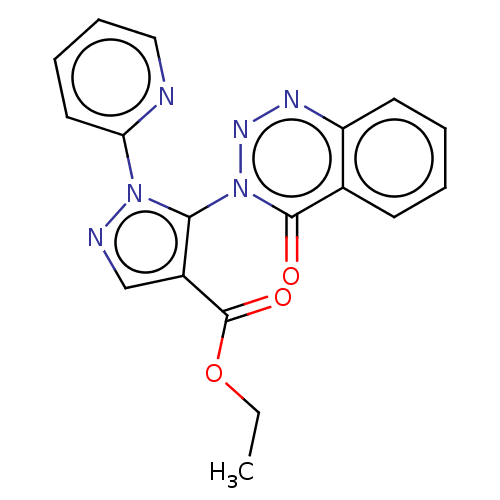
(CHEMBL4437353)Show SMILES CCOC(=O)c1cnn(c1-n1nnc2ccccc2c1=O)-c1ccccn1 |(5.47,-2.71,;7.01,-2.71,;7.77,-4.05,;9.31,-4.06,;9.64,-2.55,;10.45,-5.09,;11.96,-4.78,;12.73,-6.11,;11.69,-7.25,;10.29,-6.62,;8.96,-7.39,;8.96,-8.94,;7.61,-9.71,;6.28,-8.94,;4.95,-9.71,;3.62,-8.94,;3.62,-7.4,;4.95,-6.63,;6.29,-7.39,;7.62,-6.61,;7.62,-5.07,;12.01,-8.75,;10.86,-9.78,;11.18,-11.29,;12.64,-11.77,;13.79,-10.73,;13.47,-9.23,)| Show InChI InChI=1S/C18H14N6O3/c1-2-27-18(26)13-11-20-23(15-9-5-6-10-19-15)16(13)24-17(25)12-7-3-4-8-14(12)21-22-24/h3-11H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Tecnol£gica de Pereira
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) using arachidonic acid as substrate preincubated for 20 mins followed by substrate addition measured at 1 sec int... |
Eur J Med Chem 162: 435-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.027
BindingDB Entry DOI: 10.7270/Q2DZ0CMM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data