 Found 118 hits with Last Name = 'adebayo' and Initial = 'fo'
Found 118 hits with Last Name = 'adebayo' and Initial = 'fo' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086986
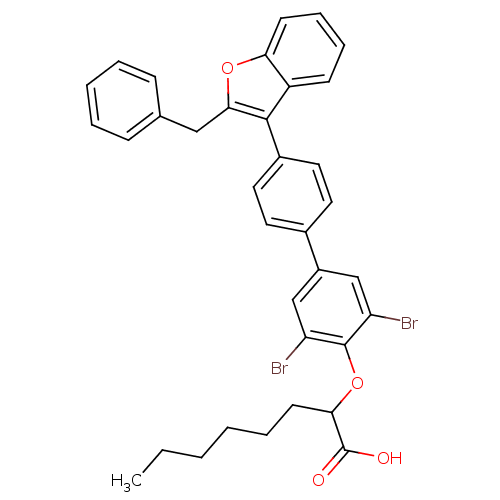
(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES CCCCCCC(Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12)C(O)=O Show InChI InChI=1S/C35H32Br2O4/c1-2-3-4-8-15-31(35(38)39)41-34-28(36)21-26(22-29(34)37)24-16-18-25(19-17-24)33-27-13-9-10-14-30(27)40-32(33)20-23-11-6-5-7-12-23/h5-7,9-14,16-19,21-22,31H,2-4,8,15,20H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086896

(4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3-bromo-bi...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H23BrO6S2/c35-28-19-24(14-17-30(28)41-43(39,40)25-15-16-26(34(37)38)29(36)20-25)22-10-12-23(13-11-22)33-27-8-4-5-9-31(27)42-32(33)18-21-6-2-1-3-7-21/h1-17,19-20,36H,18H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086972

(2-{4-[4-(2-benzyl-1-benzothiophen-3-yl)phenyl]-2,6...)Show SMILES COc1cccc(c1)-c1cc(cc(-c2cccc(OC)c2)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C43H34O5S/c1-46-34-14-8-12-31(23-34)37-25-33(26-38(43(37)48-27-41(44)45)32-13-9-15-35(24-32)47-2)29-18-20-30(21-19-29)42-36-16-6-7-17-39(36)49-40(42)22-28-10-4-3-5-11-28/h3-21,23-26H,22,27H2,1-2H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086947

(2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibromo...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H26Br2O3S/c37-29-21-27(22-30(38)35(29)41-31(36(39)40)19-23-9-3-1-4-10-23)25-15-17-26(18-16-25)34-28-13-7-8-14-32(28)42-33(34)20-24-11-5-2-6-12-24/h1-18,21-22,31H,19-20H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086950
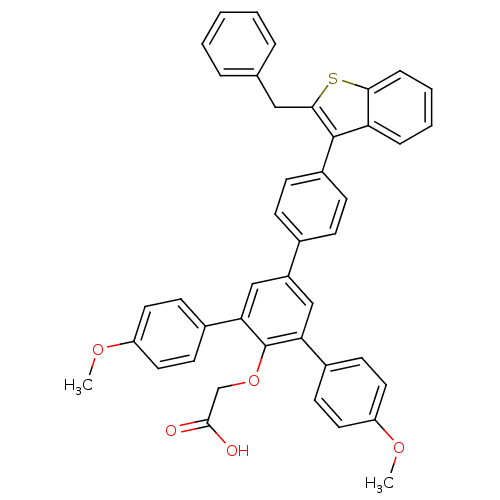
(2-[4-[4-(2-benzylbenzo[b]thiophen-3-yl)phenyl]-2,6...)Show SMILES COc1ccc(cc1)-c1cc(cc(-c2ccc(OC)cc2)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C43H34O5S/c1-46-34-20-16-30(17-21-34)37-25-33(26-38(43(37)48-27-41(44)45)31-18-22-35(47-2)23-19-31)29-12-14-32(15-13-29)42-36-10-6-7-11-39(36)49-40(42)24-28-8-4-3-5-9-28/h3-23,25-26H,24,27H2,1-2H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086911

(5-[4''-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxy...)Show SMILES OC(=O)c1cc(ccc1O)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H24O7S/c35-30-19-18-27(21-29(30)34(36)37)42(38,39)41-26-16-14-24(15-17-26)23-10-12-25(13-11-23)33-28-8-4-5-9-31(28)40-32(33)20-22-6-2-1-3-7-22/h1-19,21,35H,20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086923

(4-[4''-(2-Benzyl-benzofuran-3-yl)-3-cyclopentyl-bi...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1C1CCCC1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C39H32O7S/c40-34-24-30(19-20-31(34)39(41)42)47(43,44)46-36-21-18-29(23-33(36)27-10-4-5-11-27)26-14-16-28(17-15-26)38-32-12-6-7-13-35(32)45-37(38)22-25-8-2-1-3-9-25/h1-3,6-9,12-21,23-24,27,40H,4-5,10-11,22H2,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086970

(4-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-biphenyl-4-...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H24O6S2/c35-30-21-27(18-19-28(30)34(36)37)42(38,39)40-26-16-14-24(15-17-26)23-10-12-25(13-11-23)33-29-8-4-5-9-31(29)41-32(33)20-22-6-2-1-3-7-22/h1-19,21,35H,20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086975

(Benzothiophene derivative | CHEMBL25628 | [4-(2-Be...)Show SMILES COc1cccc(c1)-c1cc(cc(Br)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H27BrO4S/c1-40-28-11-7-10-26(19-28)30-20-27(21-31(37)36(30)41-22-34(38)39)24-14-16-25(17-15-24)35-29-12-5-6-13-32(29)42-33(35)18-23-8-3-2-4-9-23/h2-17,19-21H,18,22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086981
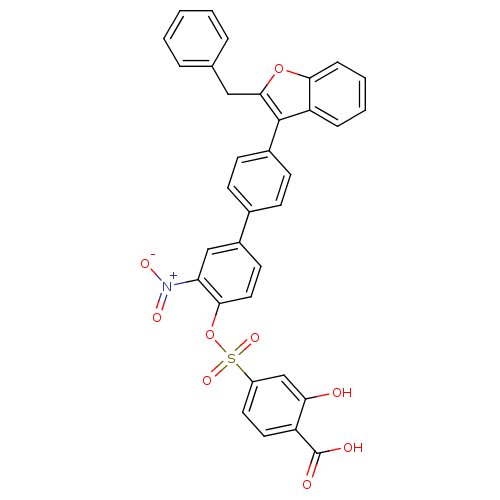
(4-[4''-(2-Benzyl-benzofuran-3-yl)-3-nitro-biphenyl...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1[N+]([O-])=O)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H23NO9S/c36-29-20-25(15-16-26(29)34(37)38)45(41,42)44-31-17-14-24(19-28(31)35(39)40)22-10-12-23(13-11-22)33-27-8-4-5-9-30(27)43-32(33)18-21-6-2-1-3-7-21/h1-17,19-20,36H,18H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086954

(CHEMBL278092 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)...)Show SMILES COc1ccc(cc1)-c1cc(cc(Br)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H27BrO4S/c1-40-28-17-15-25(16-18-28)30-20-27(21-31(37)36(30)41-22-34(38)39)24-11-13-26(14-12-24)35-29-9-5-6-10-32(29)42-33(35)19-23-7-3-2-4-8-23/h2-18,20-21H,19,22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086955
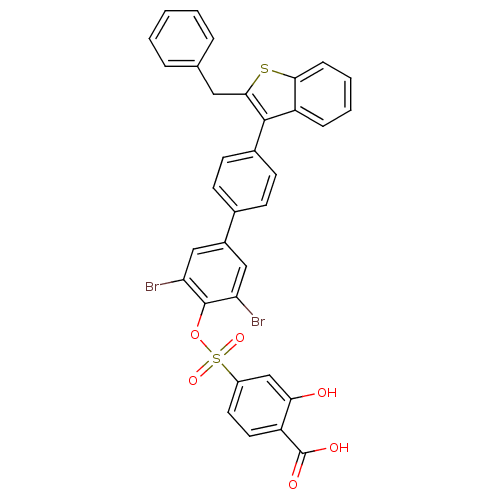
(4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibrom...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H22Br2O6S2/c35-27-17-23(18-28(36)33(27)42-44(40,41)24-14-15-25(34(38)39)29(37)19-24)21-10-12-22(13-11-21)32-26-8-4-5-9-30(26)43-31(32)16-20-6-2-1-3-7-20/h1-15,17-19,37H,16H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086951

(4-[4'-(2-Benzyl-4,5-dimethyl-thiophen-3-yl)-biphen...)Show SMILES Cc1sc(Cc2ccccc2)c(c1C)-c1ccc(cc1)-c1ccc(OS(=O)(=O)c2ccc(C(O)=O)c(O)c2)cc1 Show InChI InChI=1S/C32H26O6S2/c1-20-21(2)39-30(18-22-6-4-3-5-7-22)31(20)25-10-8-23(9-11-25)24-12-14-26(15-13-24)38-40(36,37)27-16-17-28(32(34)35)29(33)19-27/h3-17,19,33H,18H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086902

(4-[4''-(2-Benzyl-benzofuran-3-yl)-3,5-dimethyl-bip...)Show SMILES Cc1cc(cc(C)c1OS(=O)(=O)c1ccc(C(O)=O)c(O)c1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C36H28O7S/c1-22-18-27(19-23(2)35(22)43-44(40,41)28-16-17-29(36(38)39)31(37)21-28)25-12-14-26(15-13-25)34-30-10-6-7-11-32(30)42-33(34)20-24-8-4-3-5-9-24/h3-19,21,37H,20H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086892

(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C36H26Br2O4/c37-29-21-27(22-30(38)35(29)42-33(36(39)40)20-24-11-5-2-6-12-24)25-15-17-26(18-16-25)34-28-13-7-8-14-31(28)41-32(34)19-23-9-3-1-4-10-23/h1-18,21-22,33H,19-20H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086963

(4-[4'-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxys...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H24O7S/c35-30-21-27(18-19-28(30)34(36)37)42(38,39)41-26-16-14-24(15-17-26)23-10-12-25(13-11-23)33-29-8-4-5-9-31(29)40-32(33)20-22-6-2-1-3-7-22/h1-19,21,35H,20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086973

(4-[4'-(2-Benzoyl-benzofuran-3-yl)-3-cyclopentyl-bi...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1C1CCCC1)-c1ccc(cc1)-c1c(oc2ccccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C39H30O8S/c40-33-23-29(19-20-30(33)39(42)43)48(44,45)47-35-21-18-28(22-32(35)25-8-4-5-9-25)24-14-16-26(17-15-24)36-31-12-6-7-13-34(31)46-38(36)37(41)27-10-2-1-3-11-27/h1-3,6-7,10-23,25,40H,4-5,8-9H2,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086988
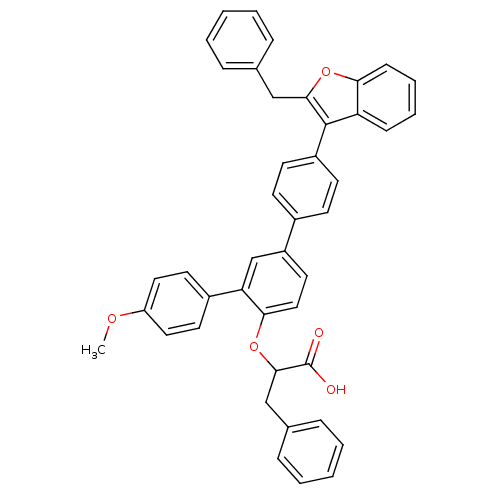
(2-[4-(2-Benzyl-benzofuran-3-yl)-4''-methoxy-[1,1';...)Show SMILES COc1ccc(cc1)-c1cc(ccc1OC(Cc1ccccc1)C(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C43H34O5/c1-46-35-23-20-32(21-24-35)37-28-34(22-25-39(37)48-41(43(44)45)27-30-12-6-3-7-13-30)31-16-18-33(19-17-31)42-36-14-8-9-15-38(36)47-40(42)26-29-10-4-2-5-11-29/h2-25,28,41H,26-27H2,1H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086952

(2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibromo...)Show SMILES OC(=O)C(CCN1C(=O)c2ccccc2C1=O)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C39H27Br2NO5S/c40-30-21-26(22-31(41)36(30)47-32(39(45)46)18-19-42-37(43)27-10-4-5-11-28(27)38(42)44)24-14-16-25(17-15-24)35-29-12-6-7-13-33(29)48-34(35)20-23-8-2-1-3-9-23/h1-17,21-22,32H,18-20H2,(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086969
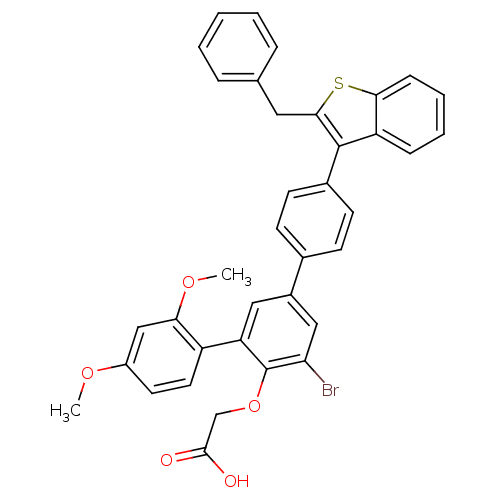
(CHEMBL24607 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)-...)Show SMILES COc1ccc(c(OC)c1)-c1cc(cc(Br)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C37H29BrO5S/c1-41-27-16-17-28(32(21-27)42-2)30-19-26(20-31(38)37(30)43-22-35(39)40)24-12-14-25(15-13-24)36-29-10-6-7-11-33(29)44-34(36)18-23-8-4-3-5-9-23/h3-17,19-21H,18,22H2,1-2H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086916
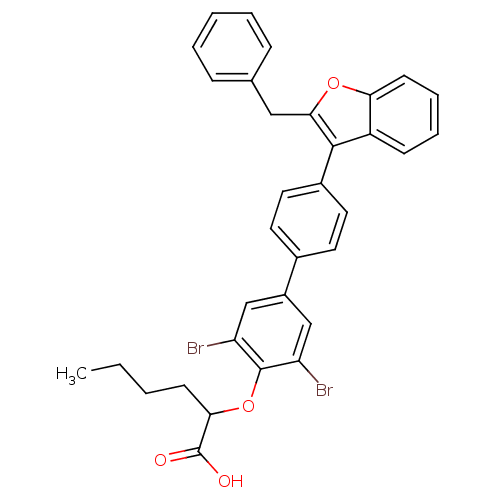
(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES CCCCC(Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12)C(O)=O Show InChI InChI=1S/C33H28Br2O4/c1-2-3-12-29(33(36)37)39-32-26(34)19-24(20-27(32)35)22-14-16-23(17-15-22)31-25-11-7-8-13-28(25)38-30(31)18-21-9-5-4-6-10-21/h4-11,13-17,19-20,29H,2-3,12,18H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086967
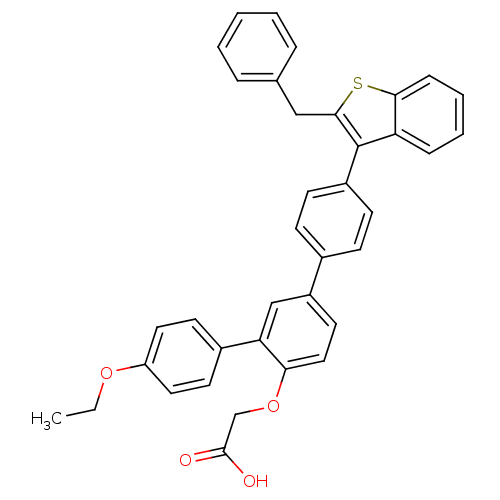
(CHEMBL284765 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)...)Show SMILES CCOc1ccc(cc1)-c1cc(ccc1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C37H30O4S/c1-2-40-30-19-16-27(17-20-30)32-23-29(18-21-33(32)41-24-36(38)39)26-12-14-28(15-13-26)37-31-10-6-7-11-34(31)42-35(37)22-25-8-4-3-5-9-25/h3-21,23H,2,22,24H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086903

(2-[4-(2-Benzyl-benzo[b]thiophen-3-yl)-4''-chloro-[...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1-c1ccc(Cl)cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C42H31ClO3S/c43-34-22-19-31(20-23-34)36-27-33(21-24-37(36)46-38(42(44)45)25-28-9-3-1-4-10-28)30-15-17-32(18-16-30)41-35-13-7-8-14-39(35)47-40(41)26-29-11-5-2-6-12-29/h1-24,27,38H,25-26H2,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086929

(2-[4-(2-Benzyl-benzo[b]thiophen-3-yl)-4''-methoxy-...)Show SMILES COc1ccc(cc1)-c1cc(ccc1OC(Cc1ccccc1)C(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C43H34O4S/c1-46-35-23-20-32(21-24-35)37-28-34(22-25-38(37)47-39(43(44)45)26-29-10-4-2-5-11-29)31-16-18-33(19-17-31)42-36-14-8-9-15-40(36)48-41(42)27-30-12-6-3-7-13-30/h2-25,28,39H,26-27H2,1H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086961
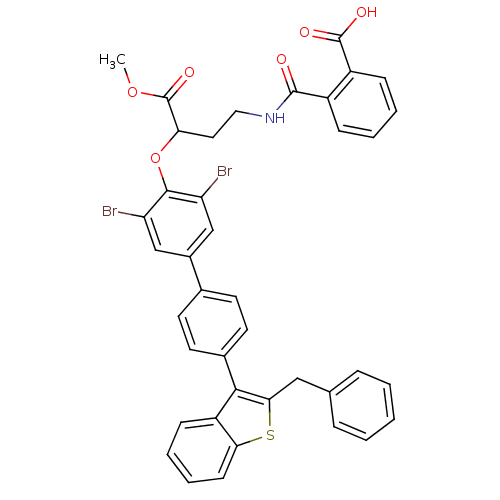
(CHEMBL25594 | N-{3-[4'-(2-Benzyl-benzo[b]thiophen-...)Show SMILES COC(=O)C(CCNC(=O)c1ccccc1C(O)=O)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C40H31Br2NO6S/c1-48-40(47)33(19-20-43-38(44)28-11-5-6-12-29(28)39(45)46)49-37-31(41)22-27(23-32(37)42)25-15-17-26(18-16-25)36-30-13-7-8-14-34(30)50-35(36)21-24-9-3-2-4-10-24/h2-18,22-23,33H,19-21H2,1H3,(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086956
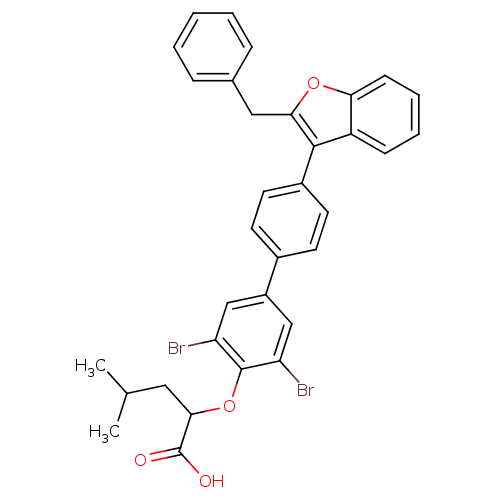
(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES CC(C)CC(Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12)C(O)=O Show InChI InChI=1S/C33H28Br2O4/c1-20(2)16-30(33(36)37)39-32-26(34)18-24(19-27(32)35)22-12-14-23(15-13-22)31-25-10-6-7-11-28(25)38-29(31)17-21-8-4-3-5-9-21/h3-15,18-20,30H,16-17H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086957

(2-[4'-(2-Benzyl-benzofuran-3-yl)-3-cyclopentyl-bip...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1C1CCCC1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C41H36O4/c42-41(43)39(26-29-13-5-2-6-14-29)45-37-24-23-33(27-35(37)31-15-7-8-16-31)30-19-21-32(22-20-30)40-34-17-9-10-18-36(34)44-38(40)25-28-11-3-1-4-12-28/h1-6,9-14,17-24,27,31,39H,7-8,15-16,25-26H2,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086953
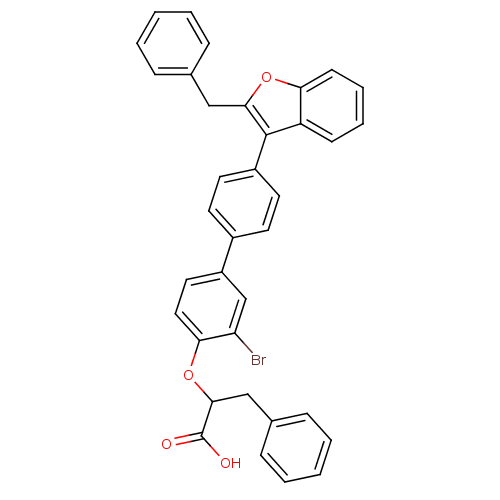
(2-[4'-(2-Benzyl-benzofuran-3-yl)-3-bromo-biphenyl-...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C36H27BrO4/c37-30-23-28(19-20-32(30)41-34(36(38)39)22-25-11-5-2-6-12-25)26-15-17-27(18-16-26)35-29-13-7-8-14-31(29)40-33(35)21-24-9-3-1-4-10-24/h1-20,23,34H,21-22H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086994
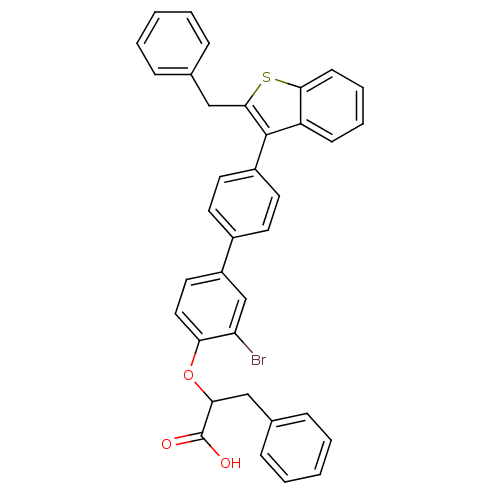
(2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-3-bromo-bip...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H27BrO3S/c37-30-23-28(19-20-31(30)40-32(36(38)39)21-24-9-3-1-4-10-24)26-15-17-27(18-16-26)35-29-13-7-8-14-33(29)41-34(35)22-25-11-5-2-6-12-25/h1-20,23,32H,21-22H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086905

(CHEMBL429385 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)...)Show SMILES COc1cccc(c1OC)-c1cc(ccc1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C37H30O5S/c1-40-32-13-8-12-28(37(32)41-2)30-22-27(19-20-31(30)42-23-35(38)39)25-15-17-26(18-16-25)36-29-11-6-7-14-33(29)43-34(36)21-24-9-4-3-5-10-24/h3-20,22H,21,23H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086914

(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dimethyl-biph...)Show SMILES Cc1cc(cc(C)c1OC(Cc1ccccc1)C(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C38H32O4/c1-25-21-31(22-26(2)37(25)42-35(38(39)40)24-28-13-7-4-8-14-28)29-17-19-30(20-18-29)36-32-15-9-10-16-33(32)41-34(36)23-27-11-5-3-6-12-27/h3-22,35H,23-24H2,1-2H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086993
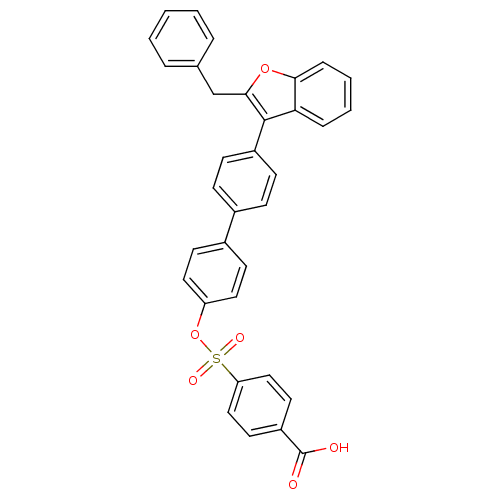
(4-[4''-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxy...)Show SMILES OC(=O)c1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H24O6S/c35-34(36)27-16-20-29(21-17-27)41(37,38)40-28-18-14-25(15-19-28)24-10-12-26(13-11-24)33-30-8-4-5-9-31(30)39-32(33)22-23-6-2-1-3-7-23/h1-21H,22H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086985
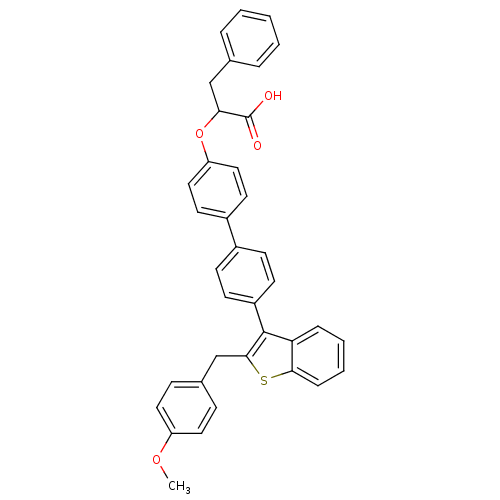
(2-{4'-[2-(4-Methoxy-benzyl)-benzo[b]thiophen-3-yl]...)Show SMILES COc1ccc(Cc2sc3ccccc3c2-c2ccc(cc2)-c2ccc(OC(Cc3ccccc3)C(O)=O)cc2)cc1 Show InChI InChI=1S/C37H30O4S/c1-40-30-19-11-26(12-20-30)24-35-36(32-9-5-6-10-34(32)42-35)29-15-13-27(14-16-29)28-17-21-31(22-18-28)41-33(37(38)39)23-25-7-3-2-4-8-25/h2-22,33H,23-24H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
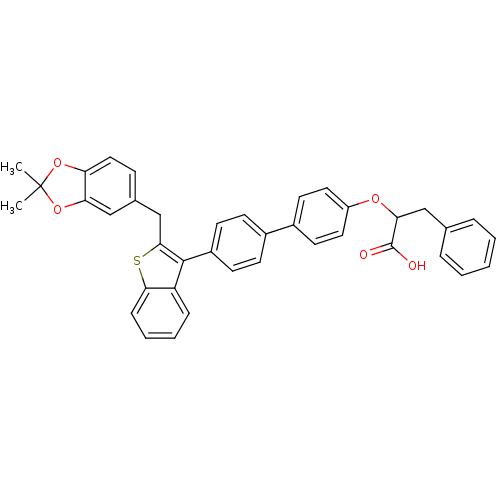
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086906
(2-{4'-[2-(2,2-Dimethyl-benzo[1,3]dioxol-5-ylmethyl...)Show SMILES CC1(C)Oc2ccc(Cc3sc4ccccc4c3-c3ccc(cc3)-c3ccc(OC(Cc4ccccc4)C(O)=O)cc3)cc2O1 Show InChI InChI=1S/C39H32O5S/c1-39(2)43-32-21-12-26(23-33(32)44-39)24-36-37(31-10-6-7-11-35(31)45-36)29-15-13-27(14-16-29)28-17-19-30(20-18-28)42-34(38(40)41)22-25-8-4-3-5-9-25/h3-21,23,34H,22,24H2,1-2H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086938

(CHEMBL24843 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)-...)Show SMILES COc1ccc(cc1)-c1cc(ccc1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H28O4S/c1-39-29-18-15-26(16-19-29)31-22-28(17-20-32(31)40-23-35(37)38)25-11-13-27(14-12-25)36-30-9-5-6-10-33(30)41-34(36)21-24-7-3-2-4-8-24/h2-20,22H,21,23H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086932
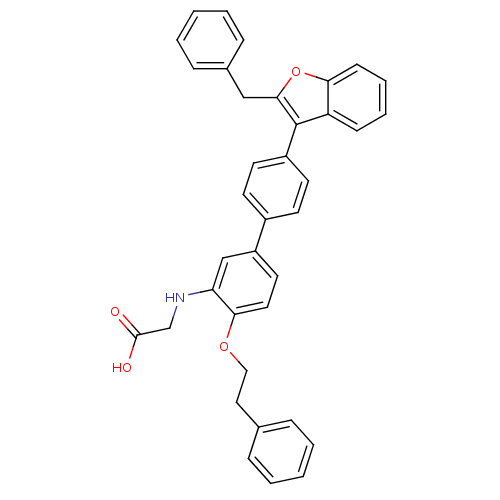
(2-(4'-(2-benzylbenzofuran-3-yl)-4-phenethoxybiphen...)Show SMILES OC(=O)CNc1cc(ccc1OCCc1ccccc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C37H31NO4/c39-36(40)25-38-32-24-30(19-20-34(32)41-22-21-26-9-3-1-4-10-26)28-15-17-29(18-16-28)37-31-13-7-8-14-33(31)42-35(37)23-27-11-5-2-6-12-27/h1-20,24,38H,21-23,25H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086977
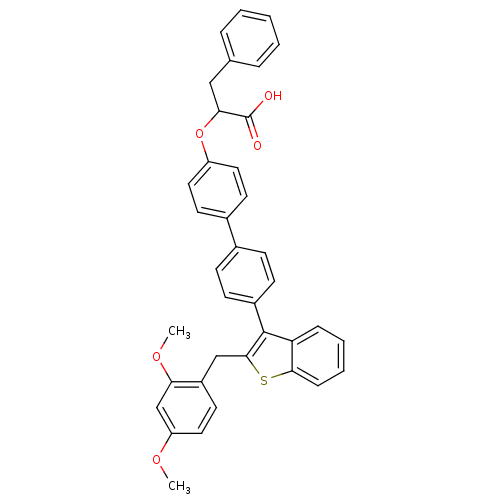
(2-{4'-[2-(2,4-Dimethoxy-benzyl)-benzo[b]thiophen-3...)Show SMILES COc1ccc(Cc2sc3ccccc3c2-c2ccc(cc2)-c2ccc(OC(Cc3ccccc3)C(O)=O)cc2)c(OC)c1 Show InChI InChI=1S/C38H32O5S/c1-41-31-21-18-29(33(24-31)42-2)23-36-37(32-10-6-7-11-35(32)44-36)28-14-12-26(13-15-28)27-16-19-30(20-17-27)43-34(38(39)40)22-25-8-4-3-5-9-25/h3-21,24,34H,22-23H2,1-2H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
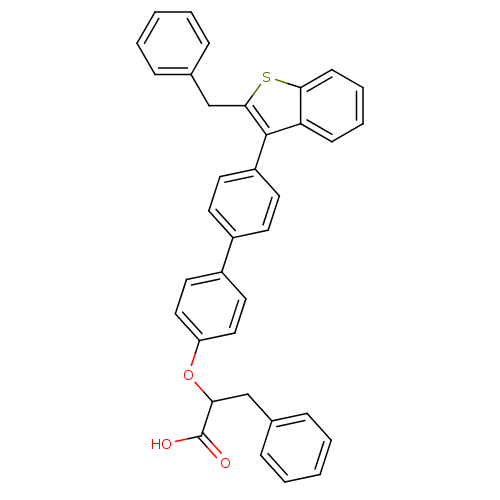
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086989
(2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-biphenyl-4-...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H28O3S/c37-36(38)32(23-25-9-3-1-4-10-25)39-30-21-19-28(20-22-30)27-15-17-29(18-16-27)35-31-13-7-8-14-33(31)40-34(35)24-26-11-5-2-6-12-26/h1-22,32H,23-24H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
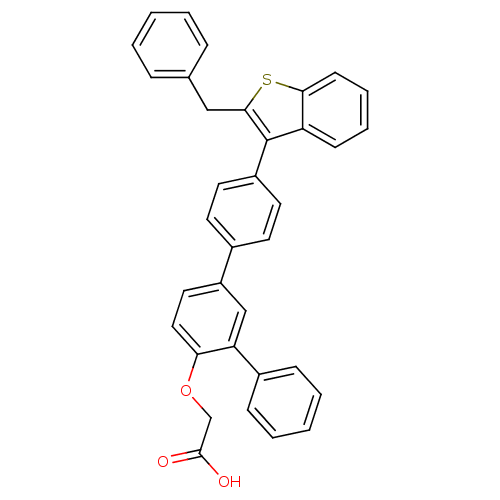
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086897
(CHEMBL24213 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)-...)Show SMILES OC(=O)COc1ccc(cc1-c1ccccc1)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C35H26O3S/c36-34(37)23-38-31-20-19-28(22-30(31)26-11-5-2-6-12-26)25-15-17-27(18-16-25)35-29-13-7-8-14-32(29)39-33(35)21-24-9-3-1-4-10-24/h1-20,22H,21,23H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
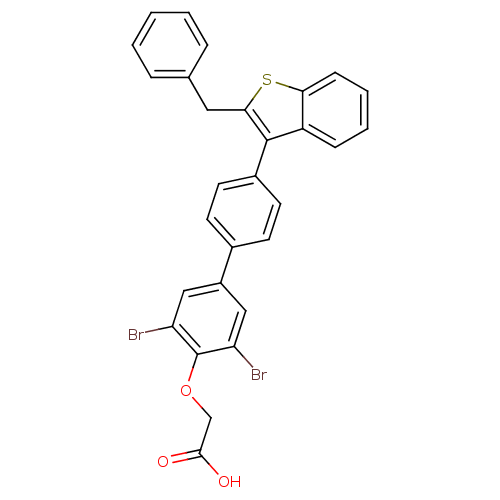
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086965
(2-(4'-(2-benzylbenzo[b]thiophen-3-yl)-3,5-dibromob...)Show SMILES OC(=O)COc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C29H20Br2O3S/c30-23-15-21(16-24(31)29(23)34-17-27(32)33)19-10-12-20(13-11-19)28-22-8-4-5-9-25(22)35-26(28)14-18-6-2-1-3-7-18/h1-13,15-16H,14,17H2,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086901
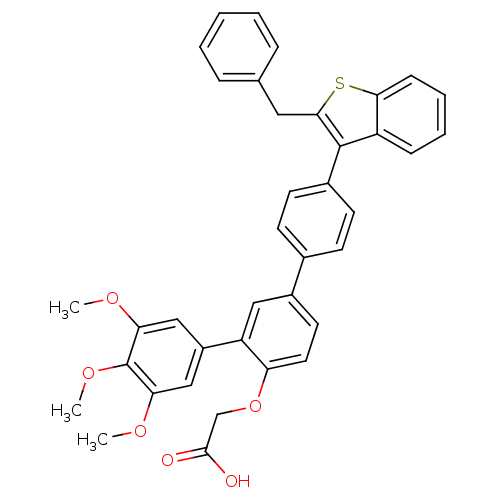
(CHEMBL25395 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)-...)Show SMILES COc1cc(cc(OC)c1OC)-c1cc(ccc1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C38H32O6S/c1-41-32-21-28(22-33(42-2)38(32)43-3)30-20-27(17-18-31(30)44-23-36(39)40)25-13-15-26(16-14-25)37-29-11-7-8-12-34(29)45-35(37)19-24-9-5-4-6-10-24/h4-18,20-22H,19,23H2,1-3H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086941
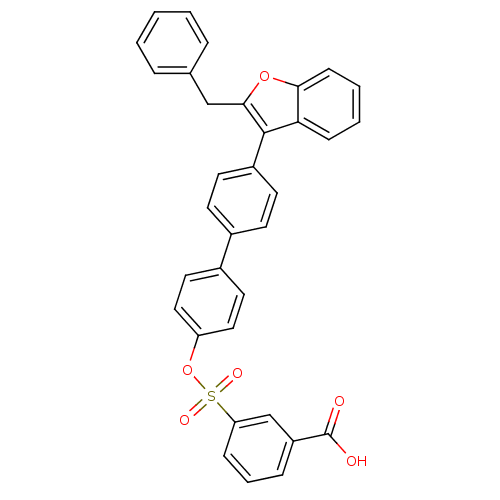
(3-[4''-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxy...)Show SMILES OC(=O)c1cccc(c1)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H24O6S/c35-34(36)27-9-6-10-29(22-27)41(37,38)40-28-19-17-25(18-20-28)24-13-15-26(16-14-24)33-30-11-4-5-12-31(30)39-32(33)21-23-7-2-1-3-8-23/h1-20,22H,21H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086974
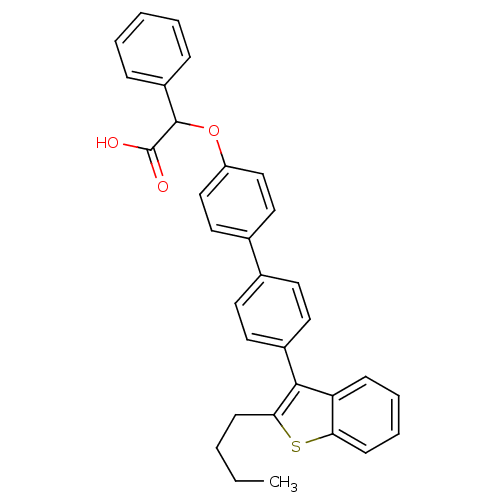
(CHEMBL24384 | [4'-(2-Butyl-benzo[b]thiophen-3-yl)-...)Show SMILES CCCCc1sc2ccccc2c1-c1ccc(cc1)-c1ccc(OC(C(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C32H28O3S/c1-2-3-12-29-30(27-11-7-8-13-28(27)36-29)24-16-14-22(15-17-24)23-18-20-26(21-19-23)35-31(32(33)34)25-9-5-4-6-10-25/h4-11,13-21,31H,2-3,12H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086931
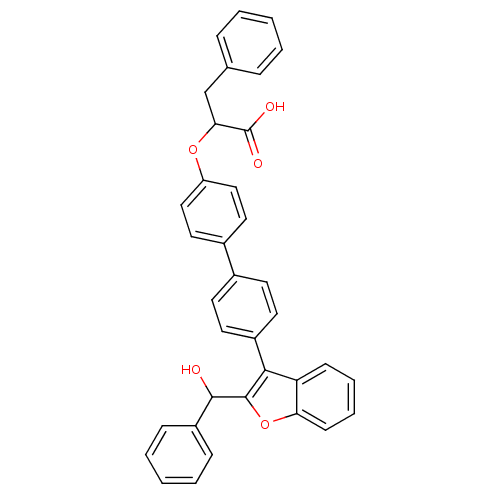
(2-{4'-[2-(Hydroxy-phenyl-methyl)-benzofuran-3-yl]-...)Show SMILES OC(c1oc2ccccc2c1-c1ccc(cc1)-c1ccc(OC(Cc2ccccc2)C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C36H28O5/c37-34(28-11-5-2-6-12-28)35-33(30-13-7-8-14-31(30)41-35)27-17-15-25(16-18-27)26-19-21-29(22-20-26)40-32(36(38)39)23-24-9-3-1-4-10-24/h1-22,32,34,37H,23H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086995
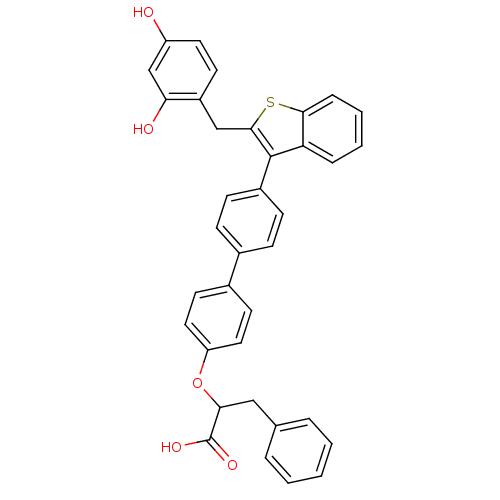
(2-{4'-[2-(2,4-Dihydroxy-benzyl)-benzo[b]thiophen-3...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccc(O)cc2O)sc2ccccc12 Show InChI InChI=1S/C36H28O5S/c37-28-17-14-27(31(38)22-28)21-34-35(30-8-4-5-9-33(30)42-34)26-12-10-24(11-13-26)25-15-18-29(19-16-25)41-32(36(39)40)20-23-6-2-1-3-7-23/h1-19,22,32,37-38H,20-21H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086939

(2-{4'-[2-(3,4-Dimethoxy-benzyl)-benzo[b]thiophen-3...)Show SMILES COc1ccc(Cc2sc3ccccc3c2-c2ccc(cc2)-c2ccc(OC(Cc3ccccc3)C(O)=O)cc2)cc1OC Show InChI InChI=1S/C38H32O5S/c1-41-32-21-12-26(23-33(32)42-2)24-36-37(31-10-6-7-11-35(31)44-36)29-15-13-27(14-16-29)28-17-19-30(20-18-28)43-34(38(39)40)22-25-8-4-3-5-9-25/h3-21,23,34H,22,24H2,1-2H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086946
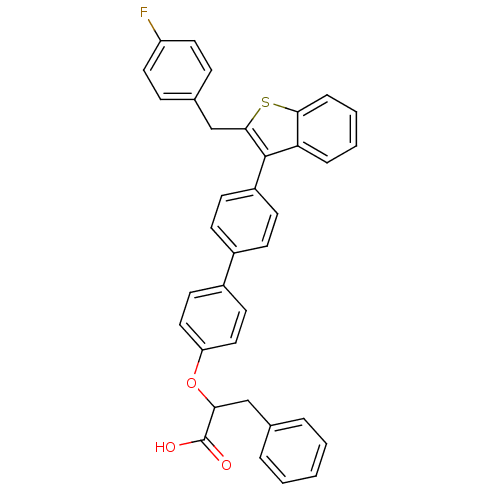
(2-{4'-[2-(4-Fluoro-benzyl)-benzo[b]thiophen-3-yl]-...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccc(F)cc2)sc2ccccc12 Show InChI InChI=1S/C36H27FO3S/c37-29-18-10-25(11-19-29)23-34-35(31-8-4-5-9-33(31)41-34)28-14-12-26(13-15-28)27-16-20-30(21-17-27)40-32(36(38)39)22-24-6-2-1-3-7-24/h1-21,32H,22-23H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086930

(2-[4'-(2-Benzyl-5-fluoro-benzofuran-3-yl)-biphenyl...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccc(F)cc12 Show InChI InChI=1S/C36H27FO4/c37-29-17-20-32-31(23-29)35(33(41-32)21-24-7-3-1-4-8-24)28-13-11-26(12-14-28)27-15-18-30(19-16-27)40-34(36(38)39)22-25-9-5-2-6-10-25/h1-20,23,34H,21-22H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086919
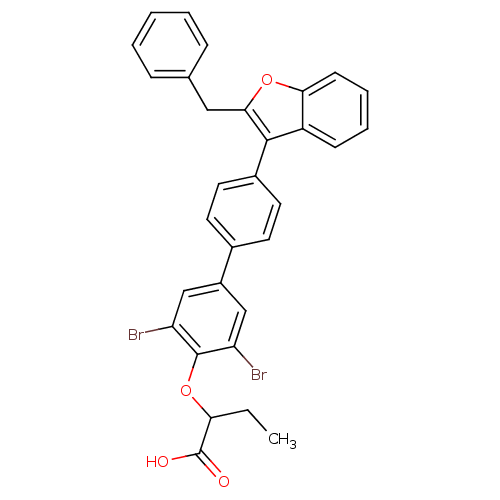
(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES CCC(Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12)C(O)=O Show InChI InChI=1S/C31H24Br2O4/c1-2-26(31(34)35)37-30-24(32)17-22(18-25(30)33)20-12-14-21(15-13-20)29-23-10-6-7-11-27(23)36-28(29)16-19-8-4-3-5-9-19/h3-15,17-18,26H,2,16H2,1H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086927
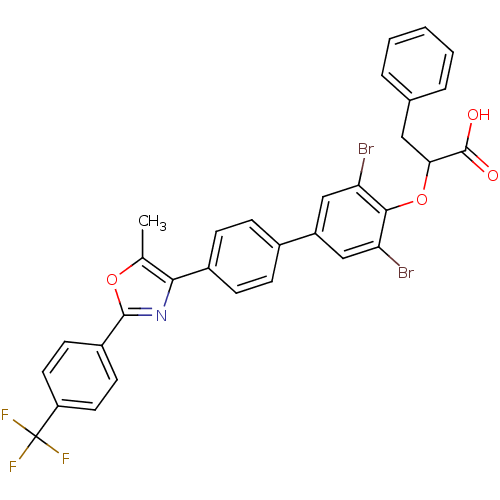
(2-{3,5-Dibromo-4''-[5-methyl-2-(4-trifluoromethyl-...)Show SMILES Cc1oc(nc1-c1ccc(cc1)-c1cc(Br)c(OC(Cc2ccccc2)C(O)=O)c(Br)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C32H22Br2F3NO4/c1-18-28(38-30(41-18)22-11-13-24(14-12-22)32(35,36)37)21-9-7-20(8-10-21)23-16-25(33)29(26(34)17-23)42-27(31(39)40)15-19-5-3-2-4-6-19/h2-14,16-17,27H,15H2,1H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data