

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608714 (CHEMBL5281897) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608719 (CHEMBL5269766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50164227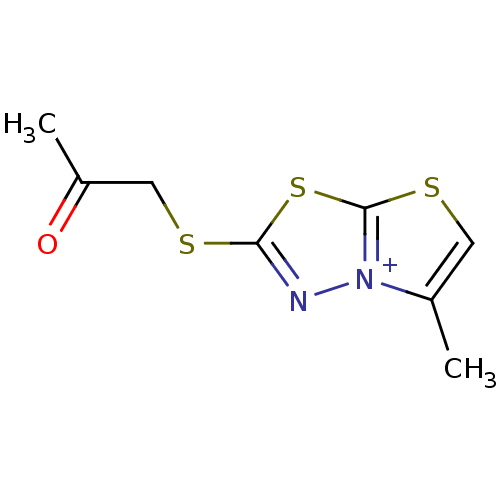 (5-Methyl-2-(2-oxo-propylsulfanyl)-thiazolo[2,3-b][...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608720 (CHEMBL5285085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM577548 (US11472838, Compound Ref. 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608718 (CHEMBL5286758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50136842 (CHEMBL152117 | GNF-Pf-5230 | N-[2-(4-Hydroxy-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386271 (CHEMBL2040983) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608713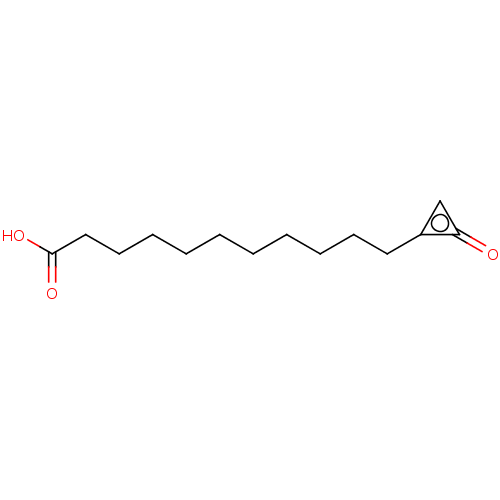 (CHEMBL5275210) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608710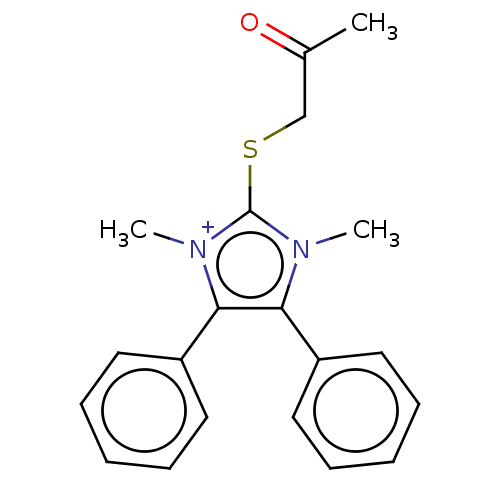 (CHEMBL5290263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50425968 (CHEMBL2314388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 551 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human FXIa assessed as S-2366 hydrolysis after 10 mins by microplate reader analysis | J Med Chem 56: 867-78 (2013) Article DOI: 10.1021/jm301338q BindingDB Entry DOI: 10.7270/Q25D8T42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608712 (CHEMBL5286863) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386267 (CHEMBL2040979) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386261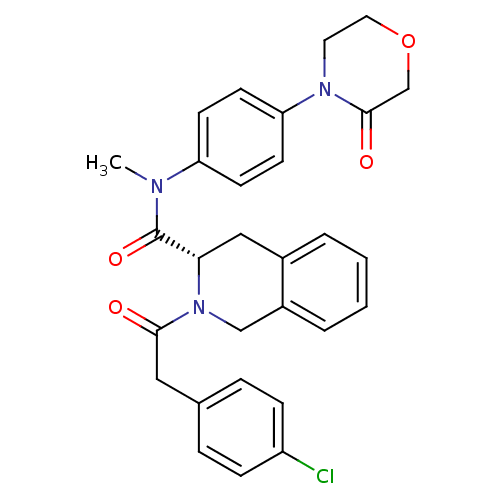 (CHEMBL2040973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in absence of exosite 2... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386253 (CHEMBL2040963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455303 (CHEMBL4218956) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using S-23666 as substrate measured after 1 min by fibrometric method | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in absence of exosite 1... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386259 (CHEMBL2040970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 30 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386270 (CHEMBL2040982) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 15 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XIII A chain [Q652E] (Homo sapiens (Human)) | BDBM50608711 (CHEMBL5290280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289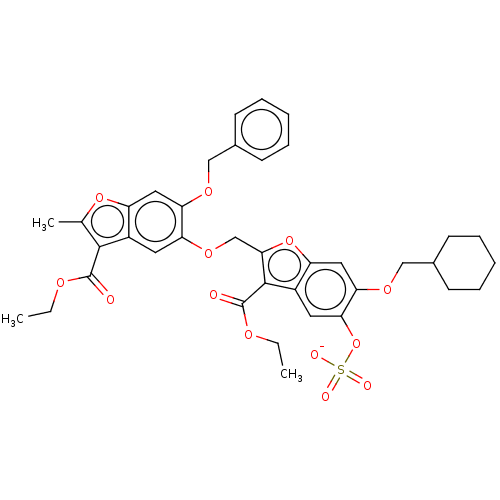 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in absence of exosite 2... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455292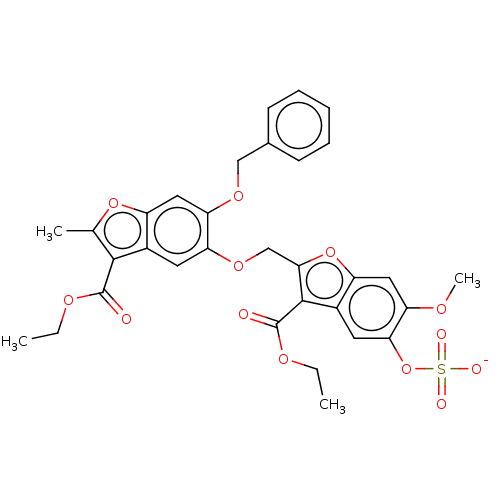 (CHEMBL4215598) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in absence of exosite 1... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455293 (CHEMBL4208688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455294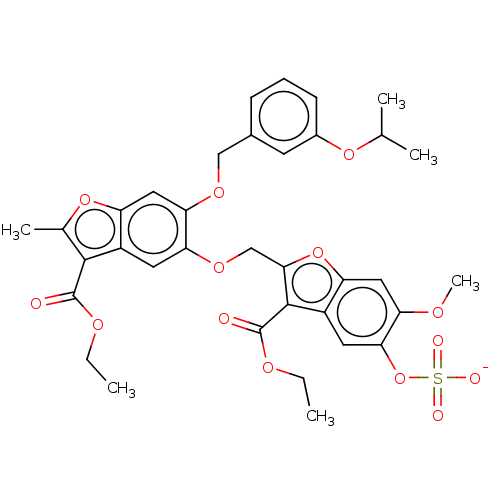 (CHEMBL4203810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455291 (CHEMBL4214824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455285 (CHEMBL4207062) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 50 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 5 uM exo... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 5 uM exo... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 10 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50260048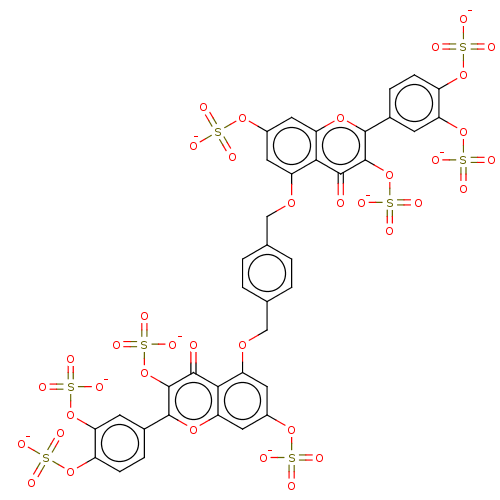 (CHEMBL4084933) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, and Institute for Structural Biology, Drug Discovery and Development, Virginia Commonwealth University , Richmond, Virginia 23219, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of human plasmin using chromogenic substrate spectrozyme PL preincubated for 5 mins followed by substrate addition measured ove... | J Med Chem 60: 641-657 (2017) Article DOI: 10.1021/acs.jmedchem.6b01474 BindingDB Entry DOI: 10.7270/Q2CF9SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 20 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 30 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50386266 (CHEMBL2040978) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human factor 10a using chromogenic spectrozyme F10a as substrate after 10 mins by spectrophotometry | Eur J Med Chem 54: 771-83 (2012) Article DOI: 10.1016/j.ejmech.2012.06.032 BindingDB Entry DOI: 10.7270/Q20P1126 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-glutamine gamma-glutamyltransferase 2 (Homo sapiens (Human)) | BDBM50608715 (CHEMBL5273935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455297 (CHEMBL4218791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455308 (CHEMBL4203401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 10 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455284 (CHEMBL4210645) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 20 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50260048 (CHEMBL4084933) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, and Institute for Structural Biology, Drug Discovery and Development, Virginia Commonwealth University , Richmond, Virginia 23219, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of human plasmin using chromogenic substrate spectrozyme PL preincubated for 5 mins followed by substrate addition measured ove... | J Med Chem 60: 641-657 (2017) Article DOI: 10.1021/acs.jmedchem.6b01474 BindingDB Entry DOI: 10.7270/Q2CF9SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455289 (CHEMBL4212514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition measured in presence of 15 uM ex... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455295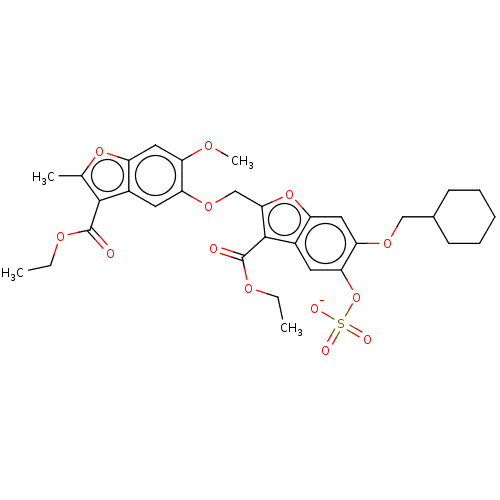 (CHEMBL4214371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50455307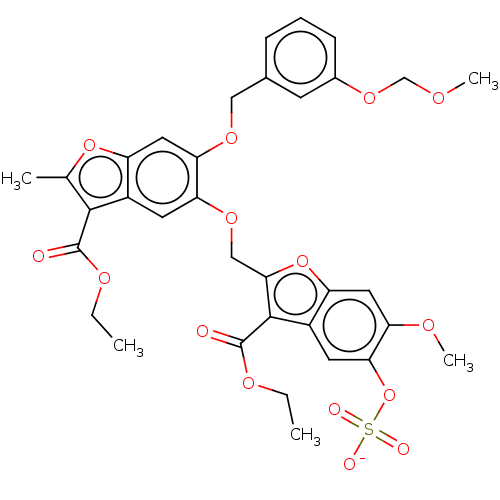 (CHEMBL4215073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Partial allosteric inhibition of human thrombin using spectrozyme TH as substrate pretreated for 10 mins followed by substrate addition by spectropho... | Bioorg Med Chem Lett 28: 1101-1105 (2018) Article DOI: 10.1016/j.bmcl.2018.01.069 BindingDB Entry DOI: 10.7270/Q2SF2ZRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50260048 (CHEMBL4084933) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, and Institute for Structural Biology, Drug Discovery and Development, Virginia Commonwealth University , Richmond, Virginia 23219, United States. Curated by ChEMBL | Assay Description Allosteric inhibition of human plasmin using chromogenic substrate spectrozyme PL preincubated for 5 mins followed by substrate addition measured ove... | J Med Chem 60: 641-657 (2017) Article DOI: 10.1021/acs.jmedchem.6b01474 BindingDB Entry DOI: 10.7270/Q2CF9SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |