 Found 583 hits with Last Name = 'alam' and Initial = 'j'
Found 583 hits with Last Name = 'alam' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D3 receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382312
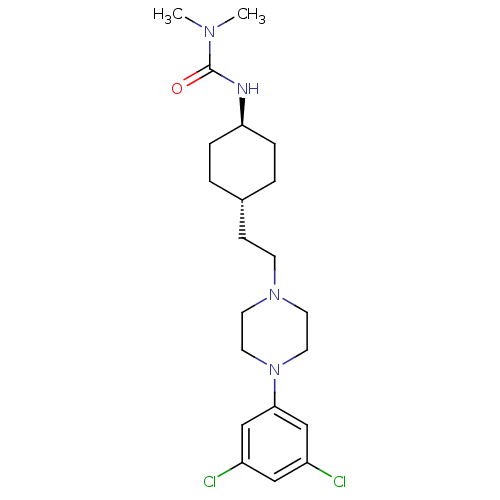
(CHEMBL2024677)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)cc(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(6.35,-23.87,;7.68,-24.65,;7.68,-26.19,;9.02,-23.88,;9.02,-22.34,;10.35,-24.65,;11.68,-23.88,;11.68,-22.34,;13.01,-21.56,;14.34,-22.34,;15.68,-21.57,;17.01,-22.35,;18.34,-21.58,;19.67,-22.36,;21,-21.6,;21.01,-20.06,;19.68,-19.28,;18.34,-20.04,;22.35,-19.3,;23.68,-20.08,;25.02,-19.32,;26.35,-20.1,;25.03,-17.78,;23.69,-17,;23.7,-15.46,;22.36,-17.76,;14.34,-23.88,;13.01,-24.64,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-19-5-3-16(4-6-19)7-8-26-9-11-27(12-10-26)20-14-17(22)13-18(23)15-20/h13-16,19H,3-12H2,1-2H3,(H,24,28)/t16-,19- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382310
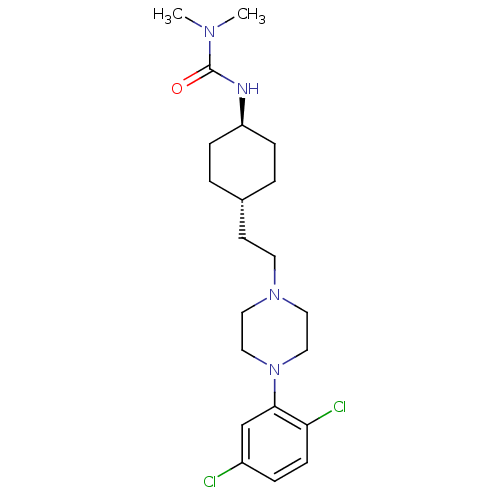
(CHEMBL2024675)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)ccc2Cl)CC1 |r,wU:6.5,wD:9.9,(23.12,-11.63,;24.45,-12.4,;24.45,-13.94,;25.79,-11.64,;25.79,-10.1,;27.12,-12.41,;28.45,-11.64,;28.45,-10.1,;29.78,-9.32,;31.11,-10.1,;32.45,-9.33,;33.78,-10.1,;35.11,-9.34,;36.44,-10.12,;37.77,-9.36,;37.78,-7.82,;36.45,-7.04,;35.11,-7.8,;39.12,-7.06,;39.13,-5.52,;40.46,-4.76,;40.46,-3.22,;41.8,-5.54,;41.79,-7.08,;40.45,-7.84,;40.44,-9.38,;31.11,-11.64,;29.78,-12.4,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-18-6-3-16(4-7-18)9-10-26-11-13-27(14-12-26)20-15-17(22)5-8-19(20)23/h5,8,15-16,18H,3-4,6-7,9-14H2,1-2H3,(H,24,28)/t16-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468037
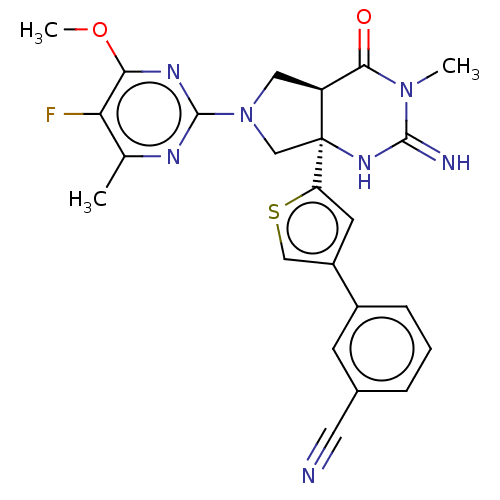
(CHEMBL4293298)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1nc(C)c(F)c(OC)n1 |r| Show InChI InChI=1S/C24H22FN7O2S/c1-13-19(25)20(34-3)29-23(28-13)32-10-17-21(33)31(2)22(27)30-24(17,12-32)18-8-16(11-35-18)15-6-4-5-14(7-15)9-26/h4-8,11,17H,10,12H2,1-3H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468040
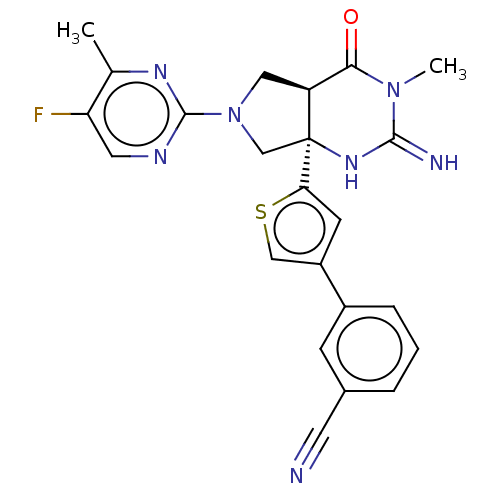
(CHEMBL4289763)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(C)n1 |r| Show InChI InChI=1S/C23H20FN7OS/c1-13-18(24)9-27-22(28-13)31-10-17-20(32)30(2)21(26)29-23(17,12-31)19-7-16(11-33-19)15-5-3-4-14(6-15)8-25/h3-7,9,11,17H,10,12H2,1-2H3,(H2,26,29)/t17-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2S receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468046

(CHEMBL4278154)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(n1)N1CCOCC1 |r| Show InChI InChI=1S/C26H25FN8O2S/c1-33-23(36)19-13-35(25-30-12-20(27)22(31-25)34-5-7-37-8-6-34)15-26(19,32-24(33)29)21-10-18(14-38-21)17-4-2-3-16(9-17)11-28/h2-4,9-10,12,14,19H,5-8,13,15H2,1H3,(H2,29,32)/t19-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468039

(CHEMBL4278329)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(OC)cc(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)18-10-31(22-27-8-16(24)9-28-22)12-23(18,29-21(30)26)19-6-15(11-34-19)14-3-13(7-25)4-17(5-14)33-2/h3-6,8-9,11,18H,10,12H2,1-2H3,(H2,26,29)/t18-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468048

(CHEMBL4280271)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(Cl)c1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C21H18ClFN6OS/c1-28-18(30)16-9-29(20-25-7-15(23)8-26-20)11-21(16,27-19(28)24)17-6-13(10-31-17)12-3-2-4-14(22)5-12/h2-8,10,16H,9,11H2,1H3,(H2,24,27)/t16-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50164512
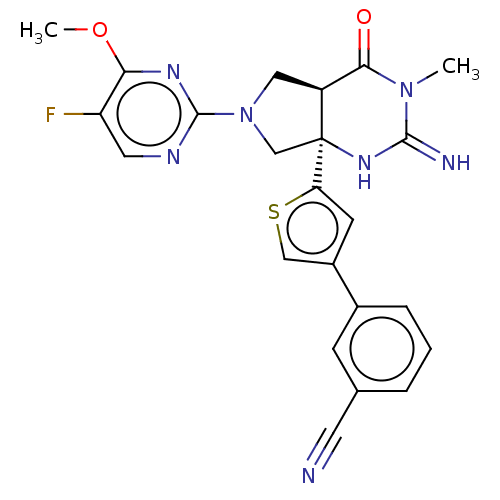
(CHEMBL3800286)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OC)n1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)16-10-31(22-27-9-17(24)19(28-22)33-2)12-23(16,29-21(30)26)18-7-15(11-34-18)14-5-3-4-13(6-14)8-25/h3-7,9,11,16H,10,12H2,1-2H3,(H2,26,29)/t16-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468044

(CHEMBL4279084)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(CC)n1 |r| Show InChI InChI=1S/C24H22FN7OS/c1-3-19-18(25)10-28-23(29-19)32-11-17-21(33)31(2)22(27)30-24(17,13-32)20-8-16(12-34-20)15-6-4-5-14(7-15)9-26/h4-8,10,12,17H,3,11,13H2,1-2H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468043

(CHEMBL4288838)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1nc(C)c(F)c(C)n1 |r| Show InChI InChI=1S/C24H22FN7OS/c1-13-20(25)14(2)29-23(28-13)32-10-18-21(33)31(3)22(27)30-24(18,12-32)19-8-17(11-34-19)16-6-4-5-15(7-16)9-26/h4-8,11,18H,10,12H2,1-3H3,(H2,27,30)/t18-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468047

(CHEMBL4278011)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OCCC)n1 |r| Show InChI InChI=1S/C25H24FN7O2S/c1-3-7-35-21-19(26)11-29-24(30-21)33-12-18-22(34)32(2)23(28)31-25(18,14-33)20-9-17(13-36-20)16-6-4-5-15(8-16)10-27/h4-6,8-9,11,13,18H,3,7,12,14H2,1-2H3,(H2,28,31)/t18-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468038
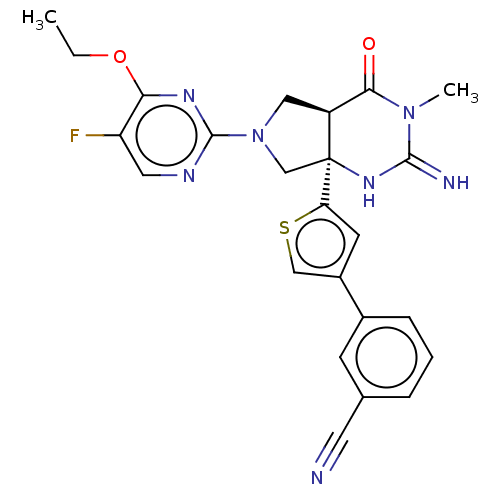
(CHEMBL4294236)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OCC)n1 |r| Show InChI InChI=1S/C24H22FN7O2S/c1-3-34-20-18(25)10-28-23(29-20)32-11-17-21(33)31(2)22(27)30-24(17,13-32)19-8-16(12-35-19)15-6-4-5-14(7-15)9-26/h4-8,10,12,17H,3,11,13H2,1-2H3,(H2,27,30)/t17-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human PAR1 in HCASMC assessed as inhibition of thrombin-induced calcium efflux |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382306

(CHEMBL2024522)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccccc2Cl)CC1 |r,wU:6.5,wD:9.9,(5.86,-.97,;7.19,-1.74,;7.19,-3.28,;8.53,-.97,;8.53,.57,;9.86,-1.74,;11.19,-.97,;11.19,.57,;12.52,1.35,;13.85,.57,;15.19,1.33,;16.52,.56,;17.85,1.33,;19.18,.55,;20.51,1.31,;20.52,2.85,;19.19,3.62,;17.85,2.86,;21.86,3.61,;21.87,5.14,;23.2,5.91,;24.54,5.13,;24.53,3.58,;23.19,2.83,;23.18,1.29,;13.85,-.97,;12.52,-1.73,)| Show InChI InChI=1S/C21H33ClN4O/c1-24(2)21(27)23-18-9-7-17(8-10-18)11-12-25-13-15-26(16-14-25)20-6-4-3-5-19(20)22/h3-6,17-18H,7-16H2,1-2H3,(H,23,27)/t17-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260518
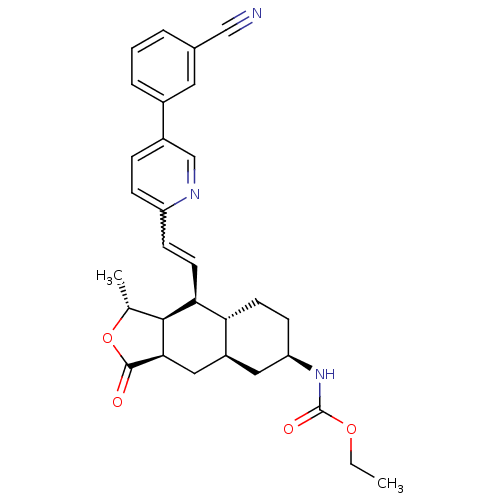
(CHEMBL442649 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(c2)C#N)C1 |r,w:21.23| Show InChI InChI=1S/C30H33N3O4/c1-3-36-30(35)33-24-10-11-25-22(14-24)15-27-28(18(2)37-29(27)34)26(25)12-9-23-8-7-21(17-32-23)20-6-4-5-19(13-20)16-31/h4-9,12-13,17-18,22,24-28H,3,10-11,14-15H2,1-2H3,(H,33,35)/t18-,22+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468045

(CHEMBL4288644)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cc(F)cc(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17F2N7OS/c1-30-19(32)17-9-31(21-27-7-16(24)8-28-21)11-22(17,29-20(30)26)18-5-14(10-33-18)13-2-12(6-25)3-15(23)4-13/h2-5,7-8,10,17H,9,11H2,1H3,(H2,26,29)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468035
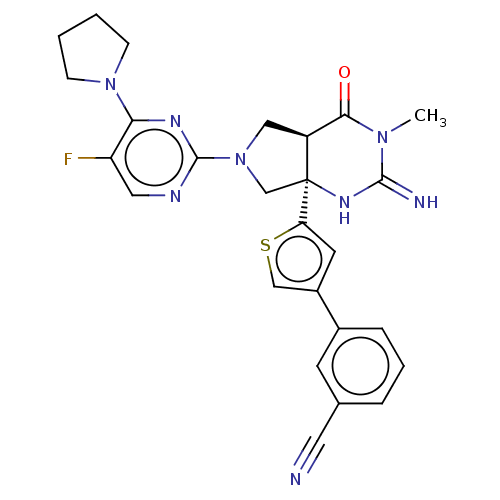
(CHEMBL4285940)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(n1)N1CCCC1 |r| Show InChI InChI=1S/C26H25FN8OS/c1-33-23(36)19-13-35(25-30-12-20(27)22(31-25)34-7-2-3-8-34)15-26(19,32-24(33)29)21-10-18(14-37-21)17-6-4-5-16(9-17)11-28/h4-6,9-10,12,14,19H,2-3,7-8,13,15H2,1H3,(H2,29,32)/t19-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382311
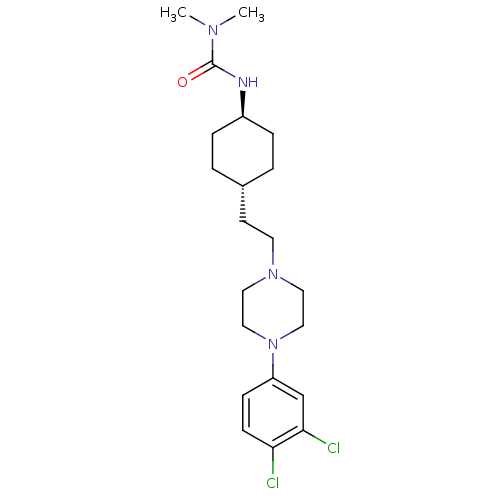
(CHEMBL2024676)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccc(Cl)c(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(-8.97,-23.7,;-7.64,-24.48,;-7.64,-26.02,;-6.31,-23.71,;-6.3,-22.17,;-4.97,-24.48,;-3.64,-23.71,;-3.64,-22.17,;-2.31,-21.39,;-.98,-22.17,;.35,-21.4,;1.69,-22.17,;3.02,-21.41,;4.35,-22.19,;5.68,-21.43,;5.69,-19.89,;4.36,-19.11,;3.02,-19.87,;7.03,-19.13,;7.04,-17.59,;8.37,-16.83,;9.71,-17.61,;11.04,-16.85,;9.7,-19.15,;11.02,-19.93,;8.36,-19.91,;-.98,-23.71,;-2.31,-24.47,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-5-3-16(4-6-17)9-10-26-11-13-27(14-12-26)18-7-8-19(22)20(23)15-18/h7-8,15-17H,3-6,9-14H2,1-2H3,(H,24,28)/t16-,17- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50323373
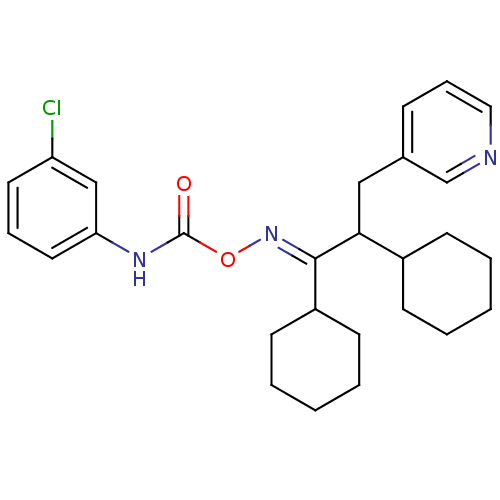
(1,2-dicyclohexyl-3-(pyridin-3-yl)propan-1-one O-3-...)Show SMILES Clc1cccc(NC(=O)O\N=C(\C(Cc2cccnc2)C2CCCCC2)C2CCCCC2)c1 Show InChI InChI=1S/C27H34ClN3O2/c28-23-14-7-15-24(18-23)30-27(32)33-31-26(22-12-5-2-6-13-22)25(21-10-3-1-4-11-21)17-20-9-8-16-29-19-20/h7-9,14-16,18-19,21-22,25H,1-6,10-13,17H2,(H,30,32)/b31-26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat mGLUR5 |
Bioorg Med Chem Lett 20: 4371-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.075
BindingDB Entry DOI: 10.7270/Q2G1611F |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261116
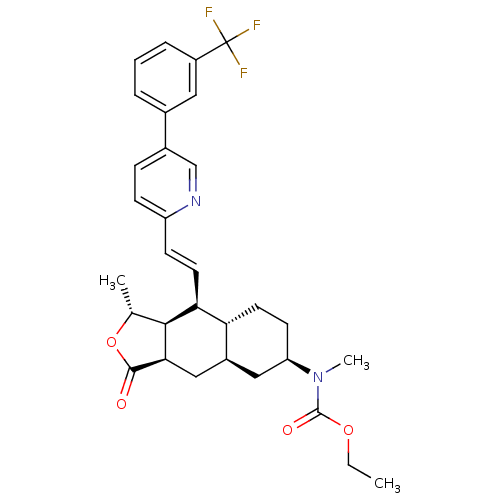
(CHEMBL453277 | ethyl methyl((1R,3aR,4aR,6R,8aR,9S,...)Show SMILES CCOC(=O)N(C)[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(c2)C(F)(F)F)C1 |r| Show InChI InChI=1S/C31H35F3N2O4/c1-4-39-30(38)36(3)24-11-13-25-21(15-24)16-27-28(18(2)40-29(27)37)26(25)12-10-23-9-8-20(17-35-23)19-6-5-7-22(14-19)31(32,33)34/h5-10,12,14,17-18,21,24-28H,4,11,13,15-16H2,1-3H3/b12-10+/t18-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50468036

(CHEMBL4286732)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1ccc(F)c(c1)C#N)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H17F2N7OS/c1-30-19(32)16-9-31(21-27-7-15(23)8-28-21)11-22(16,29-20(30)26)18-5-14(10-33-18)12-2-3-17(24)13(4-12)6-25/h2-5,7-8,10,16H,9,11H2,1H3,(H2,26,29)/t16-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398693
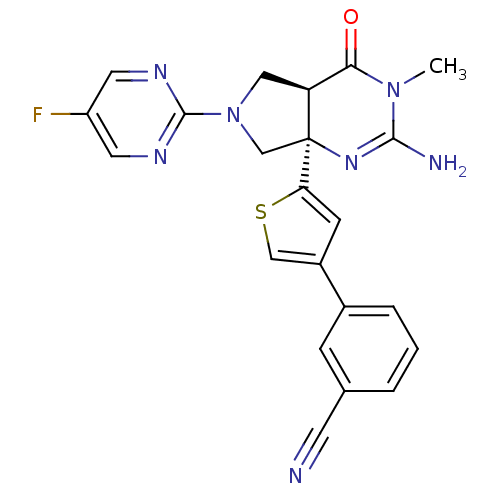
(CHEMBL2178718)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ncc(F)cn1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C22H18FN7OS/c1-29-19(31)17-10-30(21-26-8-16(23)9-27-21)12-22(17,28-20(29)25)18-6-15(11-32-18)14-4-2-3-13(5-14)7-24/h2-6,8-9,11,17H,10,12H2,1H3,(H2,25,28)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261114
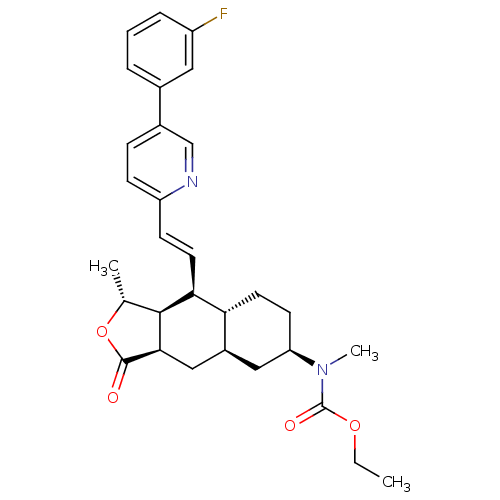
(CHEMBL500551 | Ethyl [(1R,3aR,4aR,6R,8aR,9S,9aS)-9...)Show SMILES CCOC(=O)N(C)[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 |r| Show InChI InChI=1S/C30H35FN2O4/c1-4-36-30(35)33(3)24-11-13-25-21(15-24)16-27-28(18(2)37-29(27)34)26(25)12-10-23-9-8-20(17-32-23)19-6-5-7-22(31)14-19/h5-10,12,14,17-18,21,24-28H,4,11,13,15-16H2,1-3H3/b12-10+/t18-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222015
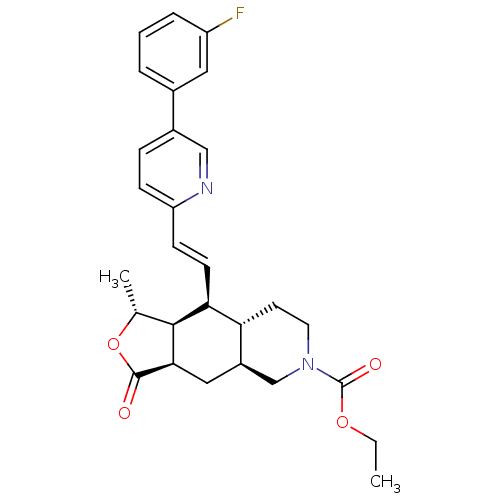
((1R,3aR,4aS,8aS,9S,9aS)-decahydro-1-methyl-3-oxo-9...)Show SMILES CCOC(=O)N1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 Show InChI InChI=1S/C28H31FN2O4/c1-3-34-28(33)31-12-11-23-20(16-31)14-25-26(17(2)35-27(25)32)24(23)10-9-22-8-7-19(15-30-22)18-5-4-6-21(29)13-18/h4-10,13,15,17,20,23-26H,3,11-12,14,16H2,1-2H3/b10-9+/t17-,20-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261061
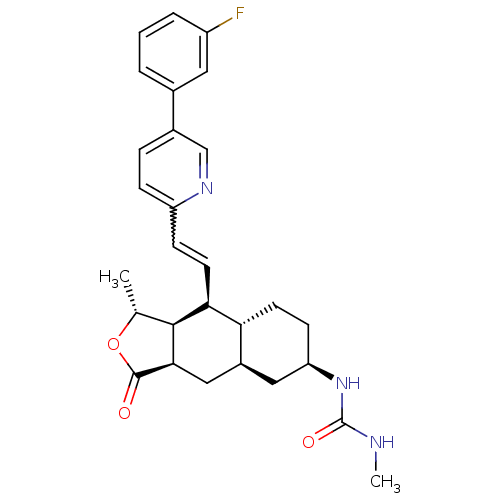
(CHEMBL493633 | N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E...)Show SMILES CNC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:20.22| Show InChI InChI=1S/C28H32FN3O3/c1-16-26-24(11-8-21-7-6-18(15-31-21)17-4-3-5-20(29)12-17)23-10-9-22(32-28(34)30-2)13-19(23)14-25(26)27(33)35-16/h3-8,11-12,15-16,19,22-26H,9-10,13-14H2,1-2H3,(H2,30,32,34)/t16-,19+,22-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261062
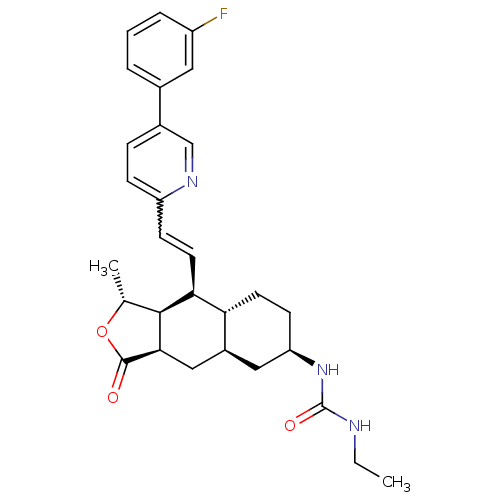
(1-ethyl-3-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E)-2-(5-...)Show SMILES CCNC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H34FN3O3/c1-3-31-29(35)33-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)34)25(24)12-9-22-8-7-19(16-32-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H2,31,33,35)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50139455
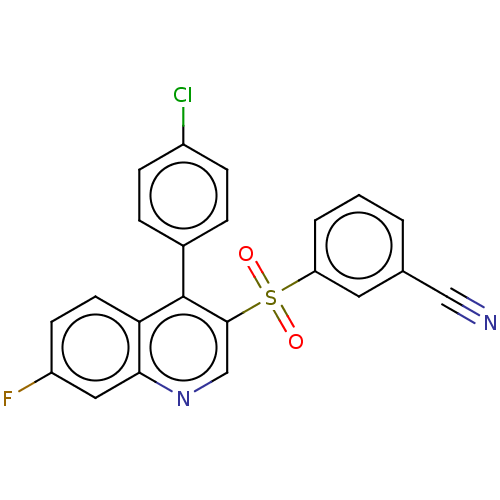
(CHEMBL3765114)Show SMILES Fc1ccc2c(-c3ccc(Cl)cc3)c(cnc2c1)S(=O)(=O)c1cccc(c1)C#N Show InChI InChI=1S/C22H12ClFN2O2S/c23-16-6-4-15(5-7-16)22-19-9-8-17(24)11-20(19)26-13-21(22)29(27,28)18-3-1-2-14(10-18)12-25/h1-11,13H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]R21412 from recombinant human mGluR5a expressed in human A18 cell membrane homogenate |
J Med Chem 60: 2470-2484 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01858
BindingDB Entry DOI: 10.7270/Q2RV0R05 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382307

(CHEMBL2024523)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(24.62,.37,;25.95,-.4,;25.95,-1.94,;27.29,.37,;27.29,1.91,;28.62,-.41,;29.95,.37,;29.95,1.91,;31.28,2.68,;32.61,1.91,;33.95,2.67,;35.28,1.9,;36.61,2.66,;37.94,1.88,;39.27,2.64,;39.28,4.18,;37.95,4.96,;36.61,4.2,;40.62,4.95,;40.63,6.48,;41.96,7.24,;43.3,6.46,;43.29,4.92,;44.62,4.14,;41.95,4.16,;32.61,.37,;31.28,-.4,)| Show InChI InChI=1S/C21H33ClN4O/c1-24(2)21(27)23-19-8-6-17(7-9-19)10-11-25-12-14-26(15-13-25)20-5-3-4-18(22)16-20/h3-5,16-17,19H,6-15H2,1-2H3,(H,23,27)/t17-,19- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50236748
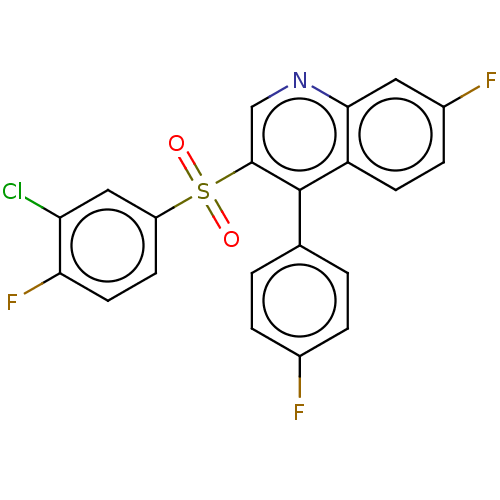
(CHEMBL4081358)Show SMILES Fc1ccc(cc1)-c1c(cnc2cc(F)ccc12)S(=O)(=O)c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C21H11ClF3NO2S/c22-17-10-15(6-8-18(17)25)29(27,28)20-11-26-19-9-14(24)5-7-16(19)21(20)12-1-3-13(23)4-2-12/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebrocortical membranes after 60 mins |
J Med Chem 60: 2470-2484 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01858
BindingDB Entry DOI: 10.7270/Q2RV0R05 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50139454

(CHEMBL3764419)Show SMILES Fc1ccc(cc1)-c1c(cnc2cc(F)ccc12)S(=O)(=O)c1ccc(Cl)c(F)c1 Show InChI InChI=1S/C21H11ClF3NO2S/c22-17-8-6-15(10-18(17)25)29(27,28)20-11-26-19-9-14(24)5-7-16(19)21(20)12-1-3-13(23)4-2-12/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from rat mGlu5 receptor expressed in rat cerebrocortical membrane measured after 1 hr |
Bioorg Med Chem Lett 26: 1249-52 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.024
BindingDB Entry DOI: 10.7270/Q2930W1N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50139455

(CHEMBL3765114)Show SMILES Fc1ccc2c(-c3ccc(Cl)cc3)c(cnc2c1)S(=O)(=O)c1cccc(c1)C#N Show InChI InChI=1S/C22H12ClFN2O2S/c23-16-6-4-15(5-7-16)22-19-9-8-17(24)11-20(19)26-13-21(22)29(27,28)18-3-1-2-14(10-18)12-25/h1-11,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from rat mGlu5 receptor expressed in rat cerebrocortical membrane measured after 1 hr |
Bioorg Med Chem Lett 26: 1249-52 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.024
BindingDB Entry DOI: 10.7270/Q2930W1N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50139455

(CHEMBL3765114)Show SMILES Fc1ccc2c(-c3ccc(Cl)cc3)c(cnc2c1)S(=O)(=O)c1cccc(c1)C#N Show InChI InChI=1S/C22H12ClFN2O2S/c23-16-6-4-15(5-7-16)22-19-9-8-17(24)11-20(19)26-13-21(22)29(27,28)18-3-1-2-14(10-18)12-25/h1-11,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]R21412 from from in Sprague-Dawley rat cerebrocortical membranes |
J Med Chem 60: 2470-2484 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01858
BindingDB Entry DOI: 10.7270/Q2RV0R05 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382309
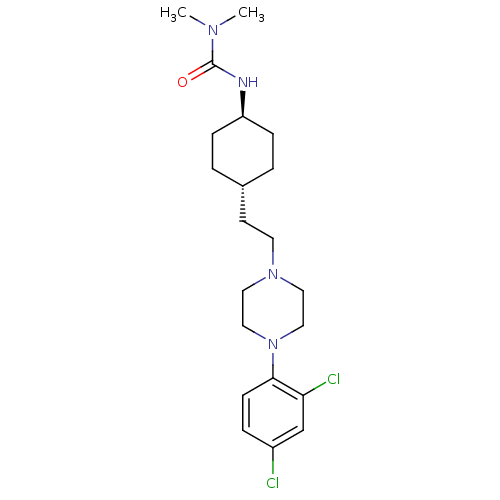
(CHEMBL2024525)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccc(Cl)cc2Cl)CC1 |r,wU:6.5,wD:9.9,(3.15,-13.27,;4.48,-14.04,;4.48,-15.58,;5.81,-13.27,;5.81,-11.73,;7.15,-14.04,;8.48,-13.27,;8.48,-11.73,;9.81,-10.95,;11.14,-11.73,;12.47,-10.96,;13.8,-11.74,;15.14,-10.97,;16.46,-11.75,;17.8,-10.99,;17.81,-9.45,;16.48,-8.67,;15.13,-9.43,;19.15,-8.69,;19.15,-7.16,;20.49,-6.39,;21.82,-7.17,;23.16,-6.41,;21.81,-8.71,;20.48,-9.47,;20.46,-11.01,;11.14,-13.27,;9.81,-14.03,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-18-6-3-16(4-7-18)9-10-26-11-13-27(14-12-26)20-8-5-17(22)15-19(20)23/h5,8,15-16,18H,3-4,6-7,9-14H2,1-2H3,(H,24,28)/t16-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2L receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260520
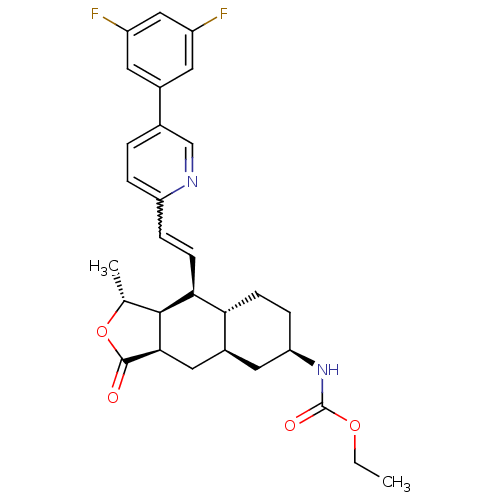
(CHEMBL458264 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cc(F)cc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H32F2N2O4/c1-3-36-29(35)33-23-7-8-24-19(12-23)13-26-27(16(2)37-28(26)34)25(24)9-6-22-5-4-17(15-32-22)18-10-20(30)14-21(31)11-18/h4-6,9-11,14-16,19,23-27H,3,7-8,12-13H2,1-2H3,(H,33,35)/t16-,19+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261117
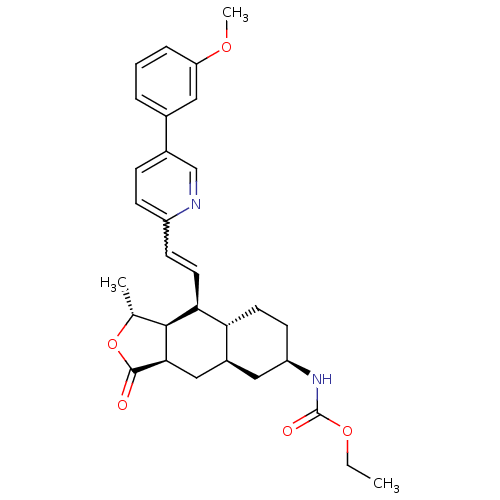
(CHEMBL446344 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(OC)c2)C1 |r,w:21.23| Show InChI InChI=1S/C30H36N2O5/c1-4-36-30(34)32-23-11-12-25-21(14-23)16-27-28(18(2)37-29(27)33)26(25)13-10-22-9-8-20(17-31-22)19-6-5-7-24(15-19)35-3/h5-10,13,15,17-18,21,23,25-28H,4,11-12,14,16H2,1-3H3,(H,32,34)/t18-,21+,23-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398687
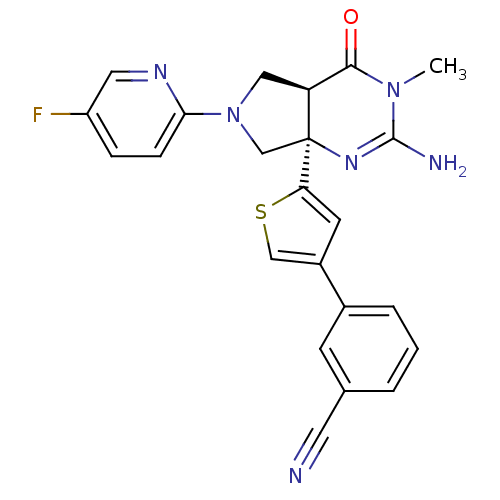
(CHEMBL2178714)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)c1ccc(F)cn1)c1cc(cs1)-c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C23H19FN6OS/c1-29-21(31)18-11-30(20-6-5-17(24)10-27-20)13-23(18,28-22(29)26)19-8-16(12-32-19)15-4-2-3-14(7-15)9-25/h2-8,10,12,18H,11,13H2,1H3,(H2,26,28)/t18-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 expressed in HEK293 cells |
J Med Chem 61: 10700-10708 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01326
BindingDB Entry DOI: 10.7270/Q2VX0K6D |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261111
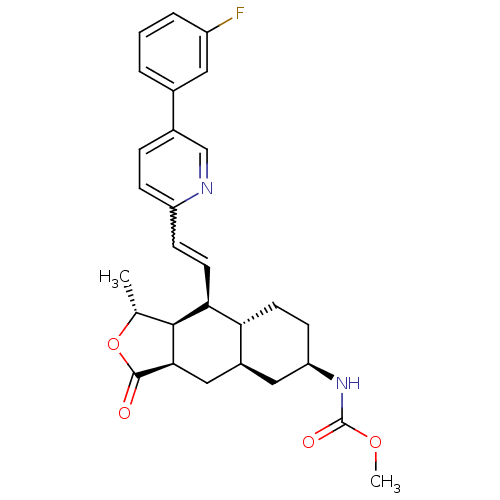
(CHEMBL493983 | methyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9...)Show SMILES COC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:20.22| Show InChI InChI=1S/C28H31FN2O4/c1-16-26-24(11-8-21-7-6-18(15-30-21)17-4-3-5-20(29)12-17)23-10-9-22(31-28(33)34-2)13-19(23)14-25(26)27(32)35-16/h3-8,11-12,15-16,19,22-26H,9-10,13-14H2,1-2H3,(H,31,33)/t16-,19+,22-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261060
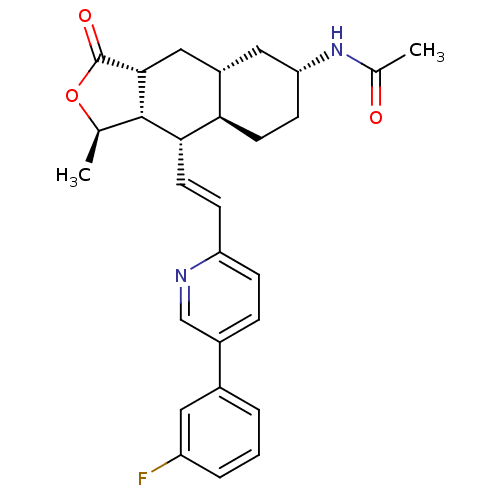
(CHEMBL493632 | N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12)NC(C)=O |r| Show InChI InChI=1S/C28H31FN2O3/c1-16-27-25(11-8-22-7-6-19(15-30-22)18-4-3-5-21(29)12-18)24-10-9-23(31-17(2)32)13-20(24)14-26(27)28(33)34-16/h3-8,11-12,15-16,20,23-27H,9-10,13-14H2,1-2H3,(H,31,32)/b11-8+/t16-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261017
(CHEMBL493792 | N-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E...)Show SMILES CCC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 |r| Show InChI InChI=1S/C29H33FN2O3/c1-3-27(33)32-23-10-11-24-20(14-23)15-26-28(17(2)35-29(26)34)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-26,28H,3,10-11,14-15H2,1-2H3,(H,32,33)/b12-9+/t17-,20+,23-,24-,25+,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261018
(CHEMBL494618 | N-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12)NC(=O)C1CC1 |r| Show InChI InChI=1S/C30H33FN2O3/c1-17-28-26(12-9-23-8-7-20(16-32-23)19-3-2-4-22(31)13-19)25-11-10-24(33-29(34)18-5-6-18)14-21(25)15-27(28)30(35)36-17/h2-4,7-9,12-13,16-18,21,24-28H,5-6,10-11,14-15H2,1H3,(H,33,34)/b12-9+/t17-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50236745
(CHEMBL4097994)Show SMILES Fc1ccc(cc1)-c1c(cnc2cc(F)ccc12)S(=O)(=O)c1cccc(c1)C#N Show InChI InChI=1S/C22H12F2N2O2S/c23-16-6-4-15(5-7-16)22-19-9-8-17(24)11-20(19)26-13-21(22)29(27,28)18-3-1-2-14(10-18)12-25/h1-11,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebrocortical membranes after 60 mins |
J Med Chem 60: 2470-2484 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01858
BindingDB Entry DOI: 10.7270/Q2RV0R05 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM86716
(CAS_63540-28-3 | CHEMBL239800 | Fenobam | NSC_2162...)Show InChI InChI=1S/C11H11ClN4O2/c1-16-6-9(17)14-10(16)15-11(18)13-8-4-2-3-7(12)5-8/h2-6,17H,1H3,(H2,13,14,15,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGLUR1 |
Bioorg Med Chem Lett 20: 4371-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.075
BindingDB Entry DOI: 10.7270/Q2G1611F |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50236742

(CHEMBL4098854)Show SMILES Fc1ccc(cc1)-c1c(cnc2c(F)cccc12)S(=O)(=O)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H11Cl2F2NO2S/c22-13-8-14(23)10-16(9-13)29(27,28)19-11-26-21-17(2-1-3-18(21)25)20(19)12-4-6-15(24)7-5-12/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebrocortical membranes after 60 mins |
J Med Chem 60: 2470-2484 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01858
BindingDB Entry DOI: 10.7270/Q2RV0R05 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50382310

(CHEMBL2024675)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)ccc2Cl)CC1 |r,wU:6.5,wD:9.9,(23.12,-11.63,;24.45,-12.4,;24.45,-13.94,;25.79,-11.64,;25.79,-10.1,;27.12,-12.41,;28.45,-11.64,;28.45,-10.1,;29.78,-9.32,;31.11,-10.1,;32.45,-9.33,;33.78,-10.1,;35.11,-9.34,;36.44,-10.12,;37.77,-9.36,;37.78,-7.82,;36.45,-7.04,;35.11,-7.8,;39.12,-7.06,;39.13,-5.52,;40.46,-4.76,;40.46,-3.22,;41.8,-5.54,;41.79,-7.08,;40.45,-7.84,;40.44,-9.38,;31.11,-11.64,;29.78,-12.4,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-18-6-3-16(4-7-18)9-10-26-11-13-27(14-12-26)20-15-17(22)5-8-19(20)23/h5,8,15-16,18H,3-4,6-7,9-14H2,1-2H3,(H,24,28)/t16-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopamine D2 receptor in rat striatal membranes |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50236711
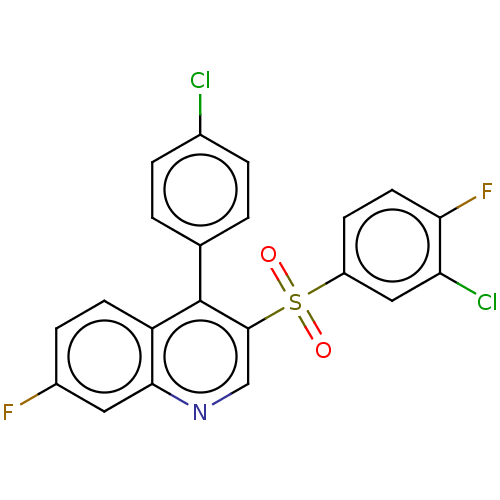
(CHEMBL4096394)Show SMILES Fc1ccc2c(-c3ccc(Cl)cc3)c(cnc2c1)S(=O)(=O)c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C21H11Cl2F2NO2S/c22-13-3-1-12(2-4-13)21-16-7-5-14(24)9-19(16)26-11-20(21)29(27,28)15-6-8-18(25)17(23)10-15/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebrocortical membranes after 60 mins |
J Med Chem 60: 2470-2484 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01858
BindingDB Entry DOI: 10.7270/Q2RV0R05 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50323380
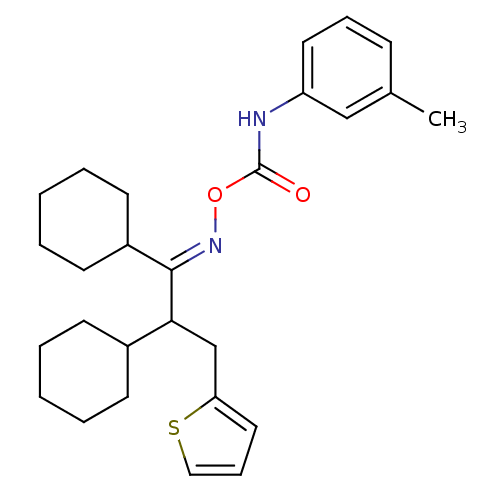
((1,2-dicyclohexyl-3-(6-thioxocyclohexa-1,3-dienyl)...)Show SMILES Cc1cccc(NC(=O)O\N=C(\C(Cc2cccs2)C2CCCCC2)C2CCCCC2)c1 Show InChI InChI=1S/C27H36N2O2S/c1-20-10-8-15-23(18-20)28-27(30)31-29-26(22-13-6-3-7-14-22)25(19-24-16-9-17-32-24)21-11-4-2-5-12-21/h8-10,15-18,21-22,25H,2-7,11-14,19H2,1H3,(H,28,30)/b29-26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat mGLUR5 |
Bioorg Med Chem Lett 20: 4371-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.075
BindingDB Entry DOI: 10.7270/Q2G1611F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data