

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50152984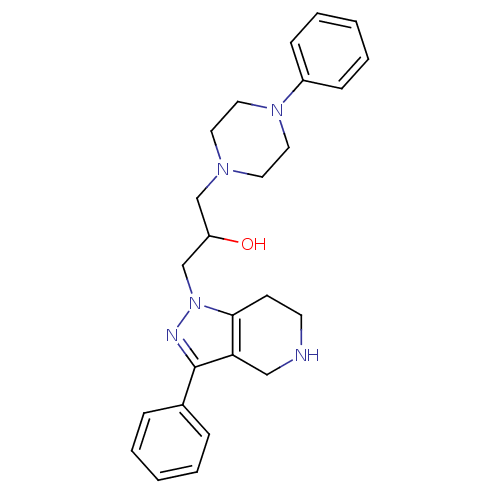 (1-(4-Phenyl-piperazin-1-yl)-3-(3-phenyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152989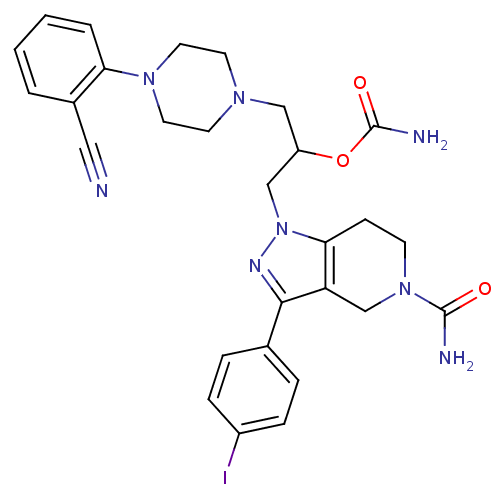 (3-(5-carbamoyl-3-(4-iodophenyl)-4,5,6,7-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152985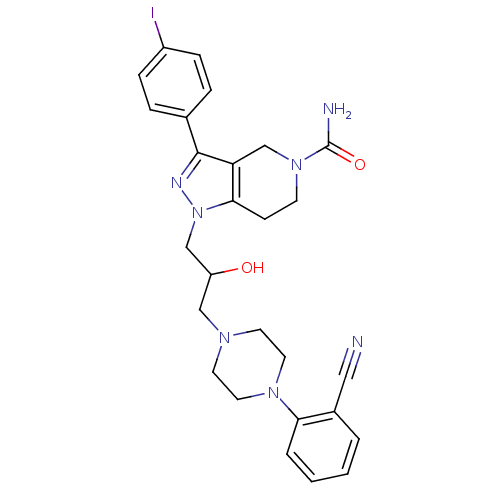 (1-{3-[4-(2-Cyano-phenyl)-piperazin-1-yl]-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152988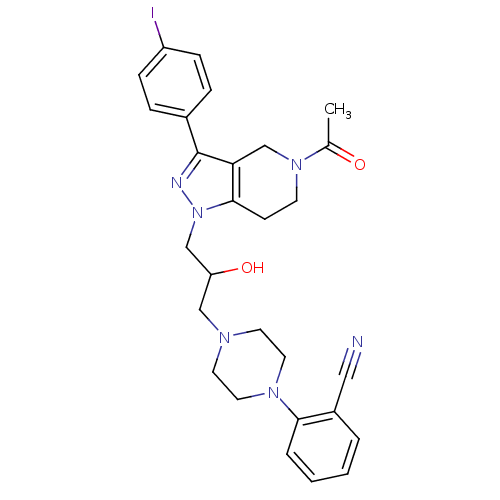 (2-(4-{3-[5-Acetyl-3-(4-iodo-phenyl)-4,5,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152990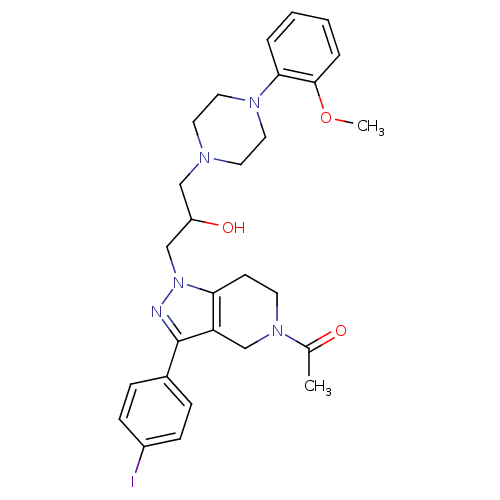 (1-[1-{2-Hydroxy-3-[4-(2-methoxy-phenyl)-piperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152986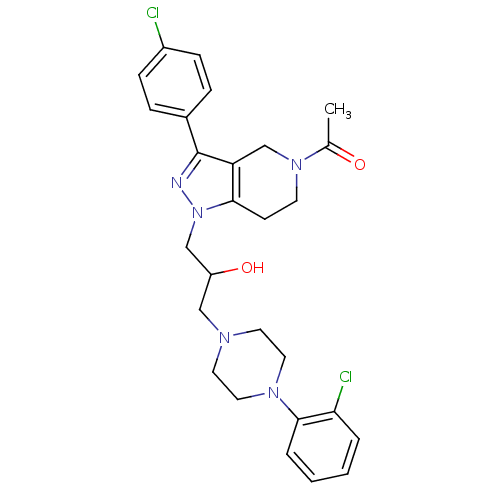 (1-(3-(4-Chloro-phenyl)-1-{3-[4-(2-chloro-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152992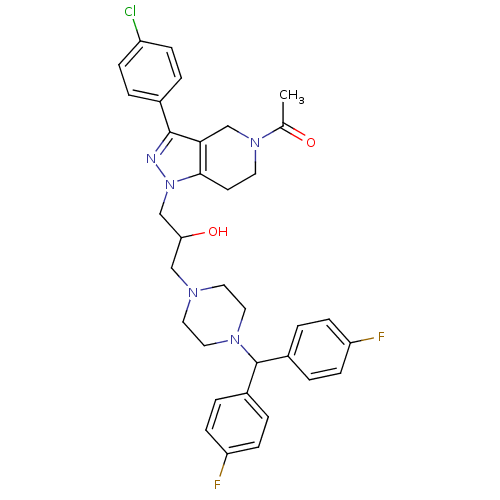 (1-[1-(3-{4-[Bis-(4-fluoro-phenyl)-methyl]-piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152991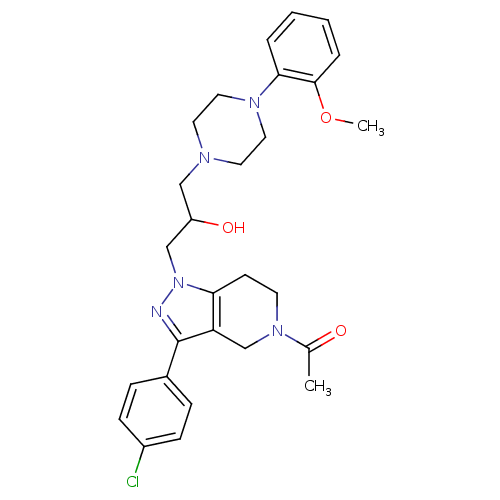 (1-(3-(4-Chloro-phenyl)-1-{2-hydroxy-3-[4-(2-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152987 (1-(3-(4-Chloro-phenyl)-1-{4-[4-(2-methoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152983 (1-[2-Hydroxy-3-(4-o-tolyl-piperazin-1-yl)-propyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory concentration against human cysteine protease cathepsin S | J Med Chem 47: 4799-801 (2004) Article DOI: 10.1021/jm0496133 BindingDB Entry DOI: 10.7270/Q28P6008 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||